(C) 2011 David R. Maddison. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The phylogeny of ground beetles of supertribe Trechitae is inferred using DNA sequences of genes that code for 28S ribosomal RNA, 18S ribosomal RNA, and wingless. Within the outgroups, austral psydrines are inferred to be monophyletic, and separate from the three genera of true Psydrina (Psydrus, Nomius, Laccocenus); the austral psydrines are formally removed from Psydrini and are treated herein as their own tribe, Moriomorphini Sloane. All three genes place Gehringia with Psydrina. Trechitae is inferred to be monophyletic, and sister to Patrobini.
Within trechites, evidence is presented that Tasmanitachoides is not a tachyine, but is instead a member of Trechini. Perileptus is a member of subtribe Trechodina. Against Erwin’s hypothesis of anillines as a polyphyletic lineage derived from the tachyine genus Paratachys, the anillines sampled are monophyletic, and not related to Paratachys. Zolini, Pogonini, Tachyina, and Xystosomina are all monophyletic, with the latter two being sister groups. The relationships of the subtribe Bembidiina were studied in greater detail. Phrypeus is only distantly related to Bembidion, and there is no evidence from sequence data that it belongs within Bembidiina. Three groups that have been recently considered to be outside of the large genus Bembidion are shown to be derived members of Bembidion, related to subgroups: Cillenus is related to the Ocydromus complex of Bembidion, Zecillenus is related to the New Zealand subgenus Zeplataphus, and Hydrium is close to subgenus Metallina. The relationships among major lineages of Trechitae are not, however, resolved with these data.
ground beetles, DNA, molecular phylogeny, Bembidiini, Trechinae, Carabidae, Trechitae,
The supertribe Trechitae comprises over 5, 300 described species (Lorenz 2005) of ground beetles. Although this is comparable to the number of mammal species (Wilson and Reeder 2005), trechites are much more poorly known. Trechites are diverse on all continents except Antarctica. Most adults of this group are relatively small (less than 10 mm in length), and include the smallest known carabids, about 0.7 mm in length (Erwin 1973; Jeannel 1963). Division of this group into suprageneric taxa varies among authors, with most North American authors favoring four tribes: Trechini and Bembidiini, with over 2, 500 species each, and the smaller groups Pogonini and Zolini, with about 85 and 55 species respectively (Lorenz 2005). Trechini includes many troglobitic species, and is most speciose in temperate areas. Bembidiini is worldwide, with many species living along bodies of water; it includes the smallest adults. This tribe includes the largest carabid genus, Bembidion, with over 1, 200 recognized species (Lorenz 2005). Most pogonines are halobiontic; the majority live in the Old World. The Zolini is a strictly south-temperate lineage, except for the monotypic genus Sinozolus from China (Deuve 1997). A brief review of the diversity within each tribe is given in Grebennikov and Maddison (2005).
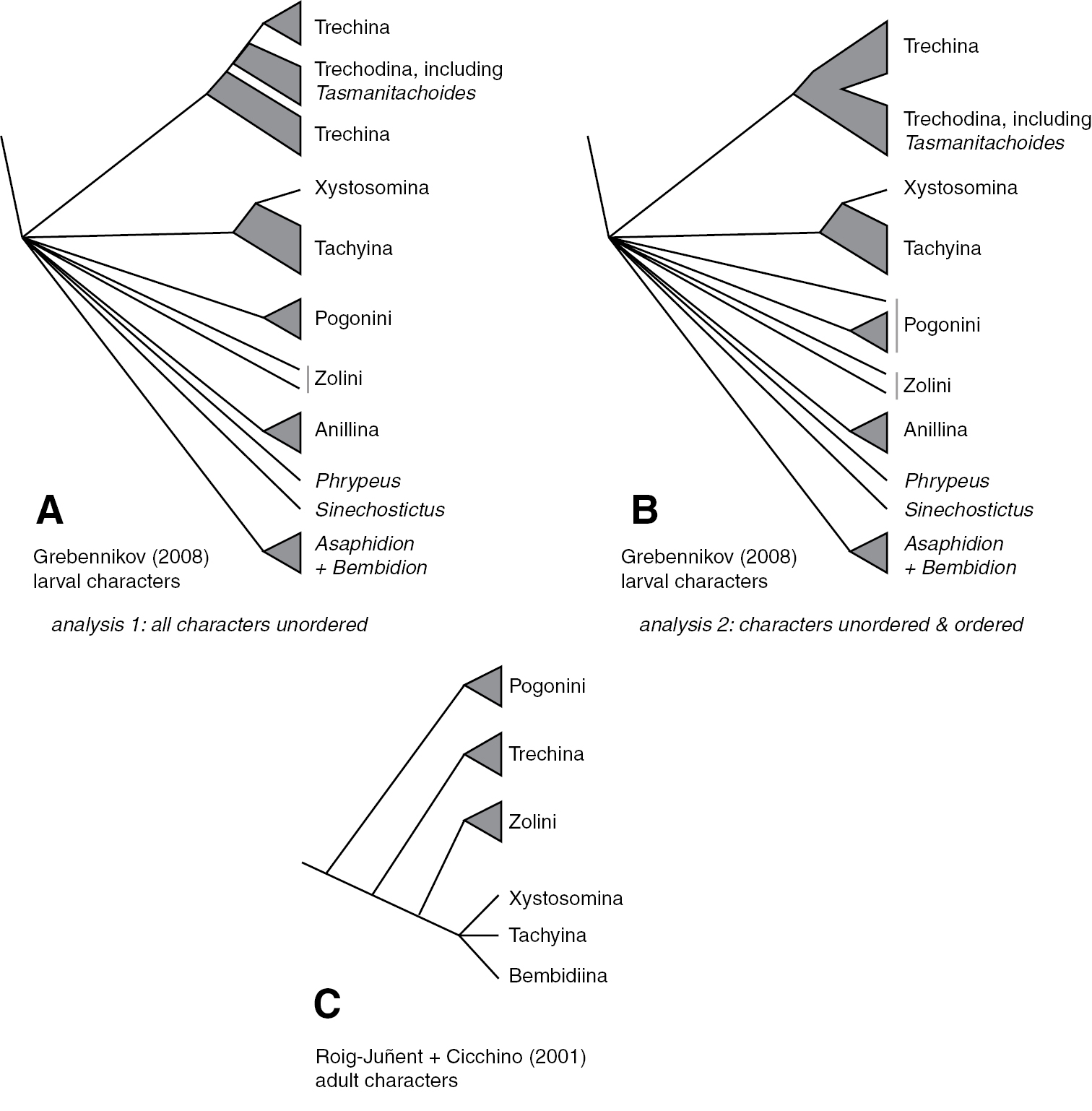
The basic structure of the phylogeny of trechites is not well known. The only explicit, modern analyses have been based upon limited characters of adult and larval structure (Grebennikov 2008; Grebennikov and Maddison 2005; Roig-Juñent and Cicchino 2001); these have inferred a few aspects of the phylogeny (Fig. 1).
Phylogenies of Trechitae from morphological studies A Strict consensus tree of most parsimonious trees from larval data, with all characters treated as unordered, from Grebennikov (2008); this is the tree presented in Grebennikov (2008: Fig. 3) B Strict consensus tree of most parsimonious trees from larval data, with some characters treated as ordered, as specified by Grebennikov (2008); this tree is not presented in that paper, but was inferred from the described conditions C “Best fit” tree presented by Roig-Juñent and Cicchino (2001) based upon adult morphological data.
Phylogenies of Trechitae from morphological studies A Strict consensus tree of most parsimonious trees from larval data, with all characters treated as unordered, from Grebennikov (2008); this is the tree presented in Grebennikov (2008: Fig. 3) B Strict consensus tree of most parsimonious trees from larval data, with some characters treated as ordered, as specified by Grebennikov (2008); this tree is not presented in that paper, but was inferred from the described conditions C “Best fit” tree presented by Roig-Juñent and Cicchino (2001) based upon adult morphological data.
We present here the first detailed examination of relationships within Trechitae based upon DNA sequences, using portions of genes for small (18S) and large (28S) subunits of ribosomal RNA, as well as the nuclear protein-coding gene wingless.
This paper has been over a decade in gestation, and some results have already been reported in other publications. For example, the discovery from the DNA sequence data reported herein of Tasmanitachoides place in Trechini rather than in Tachyina was the inspiration for Grebennikov’s search for Tasmanitachoides larvae, which as he recently reported (Grebennikov 2008) confirms its placement in Trechini.
MethodsTaxa examined. The 14 outgroup species included are listed in Table 1. Morphological data and previously collected 18S rDNA data suggests that the sister group of Trechitae is likely Patrobini (Arndt 1993; Deuve 1993; Maddison et al. 1999a; Müller 1975; Zamotajlov 2002), and we include three species of this near outgroup. More distant relatives are less clear. We include representatives of carabid groups that are of a similar grade as trechites, that is, they are members of Carabidae Conjunctae but are not members of Harpalinae or Brachininae. These include all three genera of Psydrini (s.str.), as well as six genera of Moriomorphini. The latter is the group referred to as “austral psydrines” in Maddison et al. (1999a), and includes all traditional psydrines except for Psydrini in the strict sense. In addition, as Gehringiini has been proposed to be a psydrine relative (Erwin 1985), or potentially within Trechitae (Erwin 1984), we include one member of Gehringiini, Gehringia olympica Darlington. We also include one representative of Harpalinae, Pterostichus, as the most-distant outgroup.
Outgroup taxon sampling. Four-digit numbers in entries are D.R. Maddison voucher numbers; further information on the specimens is given in the Appendix; where two numbers are listed, the sequence was formed by combining data from both specimens. Other entries are GenBank numbers of previously published sequences from Maddison et al. (1999a, 1999b), Ober (2002), and Ober and Maddison (2008).
| 18S | 28S | wingless | |
|---|---|---|---|
| Pterostichini | |||
| Pterostichus melanarius Illiger | AF002779 | AF398707 | AF398623 |
| Moriomorphini | |||
| Amblytelus curtus (Fabricius) | AF012484 | AF398683 | AF398566 |
| Mecyclothorax vulcanus (Blackburn) | AF012482 | AF398648 | AF398601 |
| Melisodera picipennis Westwood | AF012481 | AF398640 | AF398602 |
| Meonis sp. | AF398722 | AF398692 | AF398603 |
| Sitaphe parallelipennis Baehr | 0669 | 2247 | 0669 |
| Tropopterus sp. | AF012483 | 2200 | 2200 |
| Psydrini | |||
| Laccocenus ambiguus Sloane | AF012486 | AF398675 | AF398596 |
| Psydrus piceus LeConte | AF002784 | AF398684 | 1627 |
| Nomius pygmaeus (Dejean) | 0893 | AF438100 | AF437971 |
| Gehringiini | |||
| Gehringia olympica Darlington | AF012512 | AF398702 | AF398591 |
| Patrobini | |||
| Diplous californicus (Motschulsky) | AF002785 | AF398699 | AF398587 |
| Patrobus longicornis (Say) | AF002786 | AF398700 | AF398613 |
| Penetretus temporalis Bedel | 0631, 1710 | 0631 | 0631 |
| 18S | 28S | wingless | |
|---|---|---|---|
| Pterostichini | |||
| Pterostichus melanarius Illiger | AF002779 | AF398707 | AF398623 |
| Moriomorphini | |||
| Amblytelus curtus (Fabricius) | AF012484 | AF398683 | AF398566 |
| Mecyclothorax vulcanus (Blackburn) | AF012482 | AF398648 | AF398601 |
| Melisodera picipennis Westwood | AF012481 | AF398640 | AF398602 |
| Meonis sp. | AF398722 | AF398692 | AF398603 |
| Sitaphe parallelipennis Baehr | 0669 | 2247 | 0669 |
| Tropopterus sp. | AF012483 | 2200 | 2200 |
| Psydrini | |||
| Laccocenus ambiguus Sloane | AF012486 | AF398675 | AF398596 |
| Psydrus piceus LeConte | AF002784 | AF398684 | 1627 |
| Nomius pygmaeus (Dejean) | 0893 | AF438100 | AF437971 |
| Gehringiini | |||
| Gehringia olympica Darlington | AF012512 | AF398702 | AF398591 |
| Patrobini | |||
| Diplous californicus (Motschulsky) | AF002785 | AF398699 | AF398587 |
| Patrobus longicornis (Say) | AF002786 | AF398700 | AF398613 |
| Penetretus temporalis Bedel | 0631, 1710 | 0631 | 0631 |
Within Trechitae, 64 species in 40 genera are sampled, with all tribes represented, and an emphasis on subtribe Bembidiina (Table 2). The classification used here is modified version of Lorenz’s (2005), with ranks similar to those typically used in North America (e.g., Lindroth 1963). The sequences obtained for Trechus came from two different specimens from Montana; one of these is Trechus oregonensis Hatch, the other, a female, cannot be identified with certainty to species, but belongs to the Trechus chalybeus species group, to which Trechus oregonensis also belongs. In analyses combining different genes, sequences from these two individuals were combined into a chimeric taxon.
Taxon sampling of trechites. Four-digit numbers in entries are D.R. Maddison voucher numbers; further information on the specimens is given in the Appendix; where two numbers are listed, the sequence was formed by combining data from both specimens. Other entries are GenBank numbers of previously published sequences from Maddison et al. (1999a, 1999b), Ober (2002), and Ober and Maddison (2008).
| 18S | 28S | wingless | |
|---|---|---|---|
| Trechini: Trechodina | |||
| Cnides sp. | 1808 | 0691 | |
| Pachydesus sp. | 0678 | AF438112 | AF437978 |
| Perileptus areolatus (Creutzer) | 1707 | 0824 | 1707 |
| Perileptus sloanei Moore | 0767 | ||
| Thalassophilus longicornis (Sturm) | 0823 | 0823 | |
| Trechodes bipartitus (MacLeay) | 0705 | 0705 | 0705 |
| Trechodes jeanneli jeanneli Mateu | 0606 | ||
| Trechosiella sp. | 1709 | 0723 | 1709 |
| Tasmanitachoides fitzroyi (Darlington) | 1575 | 0762 | |
| Trechini: Trechina | |||
| Trechus chalybeus species group | AF002793 | ||
| Trechus oregonensis Hatch | AF398673 | 0587 | |
| Omalodera limbata Blanchard | 0571 | 0571 | 0571 |
| Homaloderodes germaini Jeannel | 1066 | ||
| Kenodactylus audouini (Guérin-Méneville) | 0670 | 0670 | |
| Paratrechus sp. | 1076 | ||
| Trechinotus flavocinctus Jeannel | 0575 | 0575 | 0575 |
| Zolini | |||
| Merizodus angusticollis Solier | AF012487 | 0453 | 0453 |
| Oopterus sp. | AF012488 | 0387 | 0387 |
| Oopterus helmsi (Sharp) | AF002787 | 0354 | 0354 |
| Sloaneana tasmaniae (Sloane) | AF002788 | 0339 | 0339 |
| Pogonini | |||
| Diplochaetus planatus (G.H. Horn) | AF002789 | AF438060 | AF437938 |
| Pogonus (Pogonus) chalceus (Marsham) | 1711 | 0679 | 0679 |
| Thalassotrechus barbarae (G.H.Horn) | 0703 | 0530 | |
| Bembidiini: Tachyina | |||
| Lymnastis sp. | 0988 | 0988 | 0988 |
| Micratopus sp. | 0605 | 0605 | |
| Paratachys vorax (LeConte) | 0410 | 0410 | |
| Elaphropus obesulus LeConte | 0411 | 0411 | 0411 |
| Elaphropus cf. nigrolimbatus Peringuey | 0761 | ||
| Elaphropus sp. 3 | 0713 | 0713 | |
| Pericompsus laetulus LeConte | AF002790 | 0429 | 0429 |
| Polyderis rufotestacea (Hayward) | 0717, 0718 | 0718 | |
| Tachys vittiger LeConte | 0760 | ||
| Tachys corax LeConte | 0604 | 0604 | |
| Tachyta nana inornata (Say) | 0573 | AF438141 | AF438002 |
| Bembidiini: Xystosomina | |||
| Erwiniana hilaris (Bates) | AF012489 | 0409 | 0409 |
| Erwiniana crassa (Erwin) | 0989 | 0989 | |
| Mioptachys flavicauda Say | 0684 | 0684 | 0684 |
| Philipis bicolor Baehr | 0592 | 0592 | |
| Bembidiini: Anillina | |||
| Anillinus (langdoni group) sp. | 0690 | 0690 | |
| Serranillus sp. | 1084 | 1084 | |
| Typhlocharis armata Coiffait | 0572, 1718 | 0572 | 0572 |
| Nesamblyops sp. | 0696 | ||
| Bembidiini: Bembidiina | |||
| Asaphidion alaskanum Wickham | 0585 | 0585 | |
| Asaphidion championi Andrewes | 0574 | ||
| Asaphidion curtum (Heyden) | AF002792 | 0267 | 0267 |
| Amerizus (Amerizus) sp. | 0576 | 0576 | |
| Ocys harpaloides (Audinet-Serville) | 0569 | 0569 | |
| Phrypeus rickseckeri Hayward | 0776 | 0692 | |
| Sinechostichus solarii (G. Müller) | 0603 | 0603 | |
| Bembidion (Antiperyphanes)sp. nr. chilense Solier | 0714 | 0714 | |
| Bembidion (Hoquedela) cf. csikii Jedlicka | 0916 | 0916 | |
| Bembidion (Cillenus) laterale (Samouelle) | 0602 | 0602 | |
| Bembidion (Notaphus) insulatum (LeConte) | 0444 | 0444 | |
| Bembidion (Bracteon) balli Lindroth | EF648613 | EF648838 | EF649474 |
| Bembidion (Odontium) coxendix Say | EF648618 | EF648837 | EF649481 |
| Bembidion (Metallina) dyschirinum LeConte | 0896 | 0896 | |
| Bembidion (Pseudoperyphus) integrum Casey | EF648659 | EF649056 | EF649609 |
| Bembidion (Bracteon) levettei carrianum Casey | EF648620 | EF648842 | EF649480 |
| Bembidion (Hydrium) levigatum Say | 0763 | 0763 | |
| Bembidion (Ocydromus) mexicanum Dejean | AF012490 | 0260 | 0262 |
| Bembidion (Phyla) obtusum Audinet-Serville | 0895 | 0895 | |
| Bembidion (Melomalus) planatum (LeConte) | 0601 | 0601 | |
| Bembidion (Bembidion) quadrimaculatum dubitans (LeConte) | 0676 | 0676 | 0676 |
| Bembidion (Zeplataphus) tairuense Bates | 0607 | 0607 | 0607 |
| Bembidion (Zecillenus) sp. | 0595 | 0595 | 0595 |
| 18S | 28S | wingless | |
|---|---|---|---|
| Trechini: Trechodina | |||
| Cnides sp. | 1808 | 0691 | |
| Pachydesus sp. | 0678 | AF438112 | AF437978 |
| Perileptus areolatus (Creutzer) | 1707 | 0824 | 1707 |
| Perileptus sloanei Moore | 0767 | ||
| Thalassophilus longicornis (Sturm) | 0823 | 0823 | |
| Trechodes bipartitus (MacLeay) | 0705 | 0705 | 0705 |
| Trechodes jeanneli jeanneli Mateu | 0606 | ||
| Trechosiella sp. | 1709 | 0723 | 1709 |
| Tasmanitachoides fitzroyi (Darlington) | 1575 | 0762 | |
| Trechini: Trechina | |||
| Trechus chalybeus species group | AF002793 | ||
| Trechus oregonensis Hatch | AF398673 | 0587 | |
| Omalodera limbata Blanchard | 0571 | 0571 | 0571 |
| Homaloderodes germaini Jeannel | 1066 | ||
| Kenodactylus audouini (Guérin-Méneville) | 0670 | 0670 | |
| Paratrechus sp. | 1076 | ||
| Trechinotus flavocinctus Jeannel | 0575 | 0575 | 0575 |
| Zolini | |||
| Merizodus angusticollis Solier | AF012487 | 0453 | 0453 |
| Oopterus sp. | AF012488 | 0387 | 0387 |
| Oopterus helmsi (Sharp) | AF002787 | 0354 | 0354 |
| Sloaneana tasmaniae (Sloane) | AF002788 | 0339 | 0339 |
| Pogonini | |||
| Diplochaetus planatus (G.H. Horn) | AF002789 | AF438060 | AF437938 |
| Pogonus (Pogonus) chalceus (Marsham) | 1711 | 0679 | 0679 |
| Thalassotrechus barbarae (G.H.Horn) | 0703 | 0530 | |
| Bembidiini: Tachyina | |||
| Lymnastis sp. | 0988 | 0988 | 0988 |
| Micratopus sp. | 0605 | 0605 | |
| Paratachys vorax (LeConte) | 0410 | 0410 | |
| Elaphropus obesulus LeConte | 0411 | 0411 | 0411 |
| Elaphropus cf. nigrolimbatus Peringuey | 0761 | ||
| Elaphropus sp. 3 | 0713 | 0713 | |
| Pericompsus laetulus LeConte | AF002790 | 0429 | 0429 |
| Polyderis rufotestacea (Hayward) | 0717, 0718 | 0718 | |
| Tachys vittiger LeConte | 0760 | ||
| Tachys corax LeConte | 0604 | 0604 | |
| Tachyta nana inornata (Say) | 0573 | AF438141 | AF438002 |
| Bembidiini: Xystosomina | |||
| Erwiniana hilaris (Bates) | AF012489 | 0409 | 0409 |
| Erwiniana crassa (Erwin) | 0989 | 0989 | |
| Mioptachys flavicauda Say | 0684 | 0684 | 0684 |
| Philipis bicolor Baehr | 0592 | 0592 | |
| Bembidiini: Anillina | |||
| Anillinus (langdoni group) sp. | 0690 | 0690 | |
| Serranillus sp. | 1084 | 1084 | |
| Typhlocharis armata Coiffait | 0572, 1718 | 0572 | 0572 |
| Nesamblyops sp. | 0696 | ||
| Bembidiini: Bembidiina | |||
| Asaphidion alaskanum Wickham | 0585 | 0585 | |
| Asaphidion championi Andrewes | 0574 | ||
| Asaphidion curtum (Heyden) | AF002792 | 0267 | 0267 |
| Amerizus (Amerizus) sp. | 0576 | 0576 | |
| Ocys harpaloides (Audinet-Serville) | 0569 | 0569 | |
| Phrypeus rickseckeri Hayward | 0776 | 0692 | |
| Sinechostichus solarii (G. Müller) | 0603 | 0603 | |
| Bembidion (Antiperyphanes)sp. nr. chilense Solier | 0714 | 0714 | |
| Bembidion (Hoquedela) cf. csikii Jedlicka | 0916 | 0916 | |
| Bembidion (Cillenus) laterale (Samouelle) | 0602 | 0602 | |
| Bembidion (Notaphus) insulatum (LeConte) | 0444 | 0444 | |
| Bembidion (Bracteon) balli Lindroth | EF648613 | EF648838 | EF649474 |
| Bembidion (Odontium) coxendix Say | EF648618 | EF648837 | EF649481 |
| Bembidion (Metallina) dyschirinum LeConte | 0896 | 0896 | |
| Bembidion (Pseudoperyphus) integrum Casey | EF648659 | EF649056 | EF649609 |
| Bembidion (Bracteon) levettei carrianum Casey | EF648620 | EF648842 | EF649480 |
| Bembidion (Hydrium) levigatum Say | 0763 | 0763 | |
| Bembidion (Ocydromus) mexicanum Dejean | AF012490 | 0260 | 0262 |
| Bembidion (Phyla) obtusum Audinet-Serville | 0895 | 0895 | |
| Bembidion (Melomalus) planatum (LeConte) | 0601 | 0601 | |
| Bembidion (Bembidion) quadrimaculatum dubitans (LeConte) | 0676 | 0676 | 0676 |
| Bembidion (Zeplataphus) tairuense Bates | 0607 | 0607 | 0607 |
| Bembidion (Zecillenus) sp. | 0595 | 0595 | 0595 |
Locality information for the specimens newly sequenced in this paper is given in the Appendix. Voucher specimens are deposited in the David Maddison voucher collection in the Oregon State Arthropod Collection at Oregon State University.
DNA sequencing. Methods for obtaining DNA sequences, including extraction methods and cycling reactions, are described in Maddison et al. (2008). Primers used are listed in Table 3; see Maddison et al. (2008) for information about original source of primer sequences. In brief, we obtained ca. 2000 bases of sequence data of 18S ribosomal DNA (18S rDNA or 18S), about 1000 bases in the D1 through D3 domains of 28S ribosomal DNA (28S rDNA, or 28S) and about 450 bases of the nuclear protein-coding gene wingless (wg). Amplified products were cleaned, quantified, and sequenced at the University of Arizona’s Genomic and Technology Core Facility using either a 3730 or 3730 XL Applied Biosystems automatic sequencer.
Primers used for DNA amplification and sequencing. Dir: direction of primer, either forward (F) or reverse (R). Syn: primer synonym. Kind: primer used for original PCR amplification and sequencing (A) or primer used only for sequencing (S). Original references for primer sequences are given in Maddison et al. (2008). Primer pairs used in earlier PCR reactions for wingless were 5wg-3wg, 5wgB-3wg2, and B5wg1-B3wg2; more recent, and reliable, reactions used the pairs wg550F-wgABRz or wg578F-wgABR.
| Gene | Primer | Syn | Dir | Kind | Sequence |
|---|---|---|---|---|---|
| 28S | LS58F | D1 | F | A | GGGAGGAAAAGAAACTAAC |
| LS998R | D3 | R | A | GCATAGTTCACCATCTTTC | |
| 18S | SS27F | 518S | F | A | TATGCTTGTCTCAAAGATTAA |
| S1893R | 18L | R | A | CACCYACGGAAACCTTGTTACGACTT | |
| SS398F | 18Sai | F | S | CCTGAGAAACGGCTACCACATC | |
| SS1054F | 760F | F | S | ATCAAGAACGAAAGT | |
| SS1090R | 18Sbi | R | S | GAGTCTCGTTCGTTATCGGA | |
| SS1554R | 909R | R | S | GTCCTGTTCCATTATTCCAT | |
| wg | wg550F | F | A | ATGCGTCAGGARTGYAARTGYCAYGGYATGTC | |
| wgAbRZ | R | A | CACTTNACYTCRCARCACCARTG | ||
| wg578F | F | A | TGCACNGTGAARACYTGCTGGATG | ||
| wgAbR | R | A | YTCGCAGCACCARTGGAA | ||
| B5wg1 | F | A | GARTGYAAGTGTCAYGGYATGTCTGG | ||
| 5wg | F | A | GARTGYAARTCYCAYGGYATGTCTGG | ||
| 5wgB | F | A | ACBTGYTGGATGCGNCTKCC | ||
| 3wg2 | R | A | CTCGCARCACCARTGGAATGTRCA | ||
| B3wg2 | R | A | ACTCGCARCACCAGTGGAATGTRCA | ||
| 3wg | R | A | ACTCGCARCACCARTGGAATGTRCA |
| Gene | Primer | Syn | Dir | Kind | Sequence |
|---|---|---|---|---|---|
| 28S | LS58F | D1 | F | A | GGGAGGAAAAGAAACTAAC |
| LS998R | D3 | R | A | GCATAGTTCACCATCTTTC | |
| 18S | SS27F | 518S | F | A | TATGCTTGTCTCAAAGATTAA |
| S1893R | 18L | R | A | CACCYACGGAAACCTTGTTACGACTT | |
| SS398F | 18Sai | F | S | CCTGAGAAACGGCTACCACATC | |
| SS1054F | 760F | F | S | ATCAAGAACGAAAGT | |
| SS1090R | 18Sbi | R | S | GAGTCTCGTTCGTTATCGGA | |
| SS1554R | 909R | R | S | GTCCTGTTCCATTATTCCAT | |
| wg | wg550F | F | A | ATGCGTCAGGARTGYAARTGYCAYGGYATGTC | |
| wgAbRZ | R | A | CACTTNACYTCRCARCACCARTG | ||
| wg578F | F | A | TGCACNGTGAARACYTGCTGGATG | ||
| wgAbR | R | A | YTCGCAGCACCARTGGAA | ||
| B5wg1 | F | A | GARTGYAAGTGTCAYGGYATGTCTGG | ||
| 5wg | F | A | GARTGYAARTCYCAYGGYATGTCTGG | ||
| 5wgB | F | A | ACBTGYTGGATGCGNCTKCC | ||
| 3wg2 | R | A | CTCGCARCACCARTGGAATGTRCA | ||
| B3wg2 | R | A | ACTCGCARCACCAGTGGAATGTRCA | ||
| 3wg | R | A | ACTCGCARCACCARTGGAATGTRCA |
Assembly of multiple chromatograms for each gene fragment and initial base calls were made with Sequencher (Gene Codes Corporation) or using Phred (Green and Ewing 2002) and Phrap (Green 1999) as orchestrated by Mesquite’s Chromaseq package (Maddison and Maddison 2009a; Maddison and Maddison 2009b), with subsequent modifications by Chromaseq and manual inspection. Multiple peaks at a single position in both reads were coded using IUPAC ambiguity codes.
Newly obtained sequences have been deposited in GenBank with accession numbers GU556024 through GU556153.
Alignment and resulting matrices. The two ribosomal genes, 18S and 28S, were aligned using ClustalW 1.8.3, with a gap opening cost of 10, gap extension of 0.1, then adjusted by eye; areas of uncertain alignment were excluded.
The amino acid translation of the wingless gene was aligned using Clustal W version 1.83 (Chenna et al. 2003) using gap opening cost of 5, gap extension cost 0.2, and a Gonnet series matrix. The central region of the wingless alignment evidently had a rich history of insertion and deletions; the alignment of this region was adjusted by eye in MacClade (Maddison and Maddison 2005). An alignment of nucleotides was then created, with the nucleotides forced to match the amino acid alignment using MacClade. There were two wingless matrices produced, one with the alignment-ambiguous region included (“all nucleotides”), and another with that region excluded (“well-aligned nucleotides”).
Phylogenetic inference. Each of the four matrices (28S, 18S, and the two wingless matrices) were subjected to parsimony, Bayesian, and maximum likelihood analyses.
Most-parsimonious trees were sought using PAUP* (Swofford 2002). For each search, 2000 replicates were conducted, each beginning with a starting tree formed by the random addition sequence option, with subsequent TBR branch rearrangement. The number of most parsimonious trees (MPTs) ranged from 15 to 502 across the four matrices, and for each matrix the MPTs were found in at least 460 of the 2000 replicates.
For parsimony bootstrap analyses in PAUP*, 1000 bootstrap replicates were conducted, each of which used a heuristic search with five replicates, each beginning with a starting tree formed by the random addition sequence option, with TBR branch rearrangement, with each replicate saving no more than 25 trees.
Models of nucleotide evolution chosen with the aid of ModelTest (Posada 2005), with the aid of PAUP* (Swofford 2002). For 18S and 28S genes, the model chosen by the Akaike Information Criterion (AIC) was a General Time Reversible rate matrix with a proportion of sites being invariant and the remainder following a gamma distribution (the GTR+I+Γ model). For the wingless gene, the GTR+I+Γ model was chosen for the region without extensive insertions and deletions, but for the indel-rich region a GTR+Γ model was preferred. When codon positions were allowed separate models, GTR+I+Γ was preferred for first positions, GTR+Γ for second positions, and HKY85+I+Γ for third positions.
Bayesian analyses were conducted using MrBayes (Huelsenbeck and Ronquist 2005). Two runs of four chains each were run for between 8 million and 30 million generations, with trees sampled every 1, 000 generations. Runs were terminated once the average standard deviation of split frequencies went below 0.01 (Huelsenbeck and Ronquist 2005). For each analysis, the trees in a burn-in period were excluded, and the majority-rule consensus tree of remaining trees was calculated by PAUP to determine Bayesian Posterior Probabilities (BPP) of clades. The burn-in period was at least 25% of the total length of the run (as only the remaining 75% were used to calculate the average standard deviation of split frequencies used as a convergence diagnostic), and extended until the likelihood scores and all parameter values reached a stable plateau, as judged by visualization tools in Tracer (Rambaut and Drummond 2004). The burn-in period ranged from 3 million generations to 25 million generations. The number of trees sampled for each analysis varied from 10, 000 to 30, 000.
Likelihood analyses of nucleotide data were conducted using RAxML version 7.0.4 (Stamatakis 2006). For each matrix, 1000 search replicates were conducted to find the maximum likelihood trees. 2000 non-parametric bootstrap replicates were used to calculate bootstrap values for groups of interest.
Several of the analyses of 18S rDNA yielded trees with the trechine Cnides, whose terminal branch was extremely long, well outside of Trechitae. As morphological data indicates definitively that Cnides is a trechite, some analyses were performed that forced it to reside within Trechitae. Two full suites of constrained analyses were conducted, one with Trechitae constrained to be monophyletic, and other with Trechini constrained to be monophyletic. For the latter, the position of Tasmanitachoides was not constrained in likelihood and parsimony analyses, allowing it to move anywhere on the tree.
All trees are shown rooted within the outgroup, arbitrarily next to Pterostichus.
Grebennikov’s (2008) larval morphological data were reanalyzed using TNT version 1.1 (Goloboff et al. 2008). Most parsimonious trees were found using the following commands: rseed[, hold 1000, xmult: hits 100 ratchet 5 norss nocss, xmult. This caused TNT to do multiple searches, each beginning with a tree with taxa added in random order, with up to 1000 trees held in memory, with each search using five cycles of ratcheting; enough searches were done until the best trees found were found 100 times. 332 equally parsimonious trees of length 140 were found.
Results of phylogenetic analysisPhylogenetic trees inferred from individual genes are shown in Figs 2–4, and from the merged matrix in Fig. 5. Support values for various hypotheses are shown in Tables 4 and 5. Summaries of supported phylogenetic hypotheses are presented in Figs 6, 7.
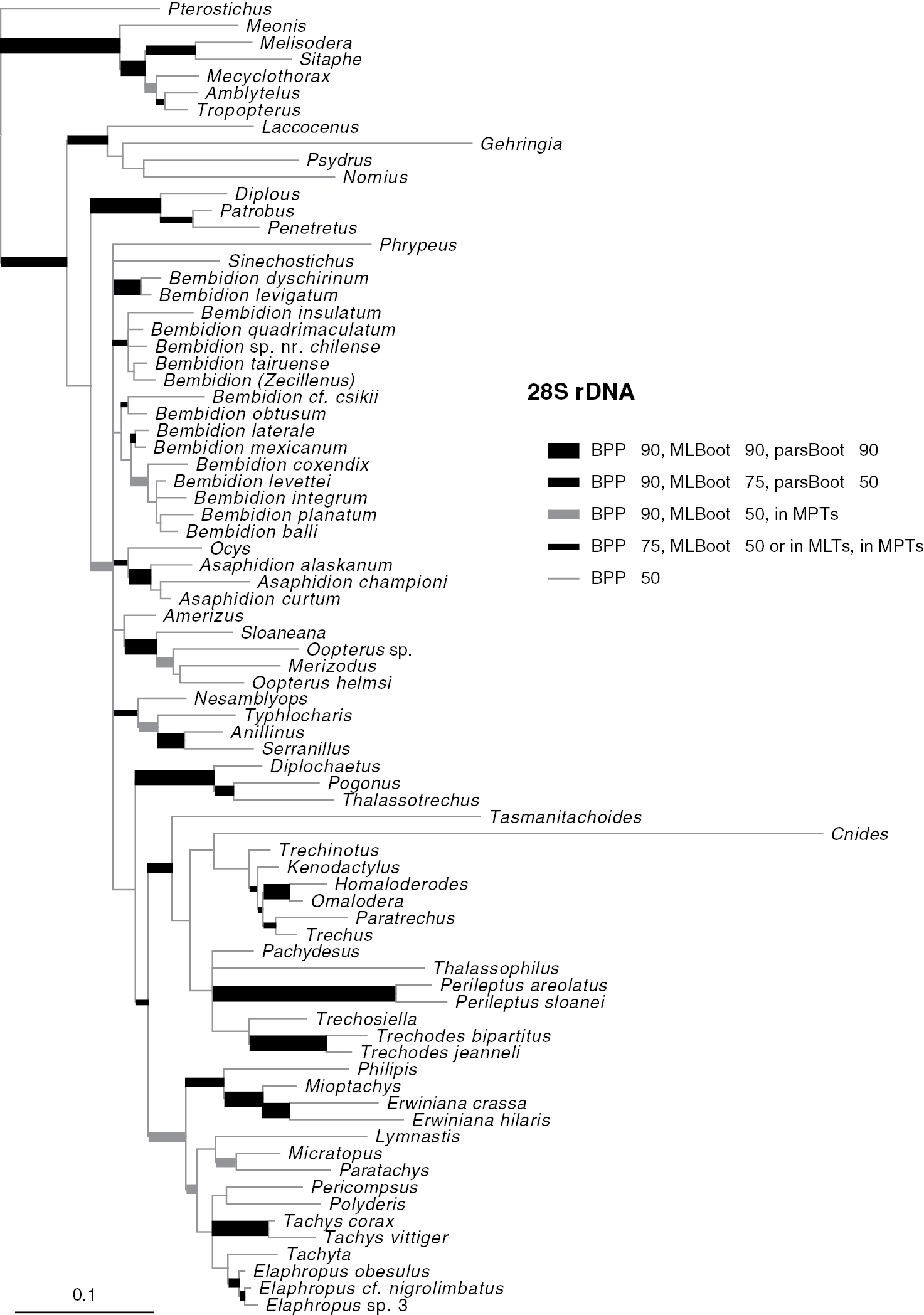
Majority-rule consensus tree of trees sampled in Bayesian analysis, with branch lengths proportional to average branch lengths across trees that contain that branch, for 28S rDNA data. Branch lengths were reconstructed by MrBayes; scale bar units are substitutions per site. Thickness and shade of branches indicate support for that clade, based upon estimated Bayesian Posterior Probability percentages (BPP), Maximum Likelihood bootstrap values (MLBoot), and parsimony bootstrap values (parsBoot).
Majority-rule consensus tree of trees sampled in Bayesian analysis, with branch lengths proportional to average branch lengths across trees that contain that branch, for 28S rDNA data. Branch lengths were reconstructed by MrBayes; scale bar units are substitutions per site. Thickness and shade of branches indicate support for that clade, based upon estimated Bayesian Posterior Probability percentages (BPP), Maximum Likelihood bootstrap values (MLBoot), and parsimony bootstrap values (parsBoot).
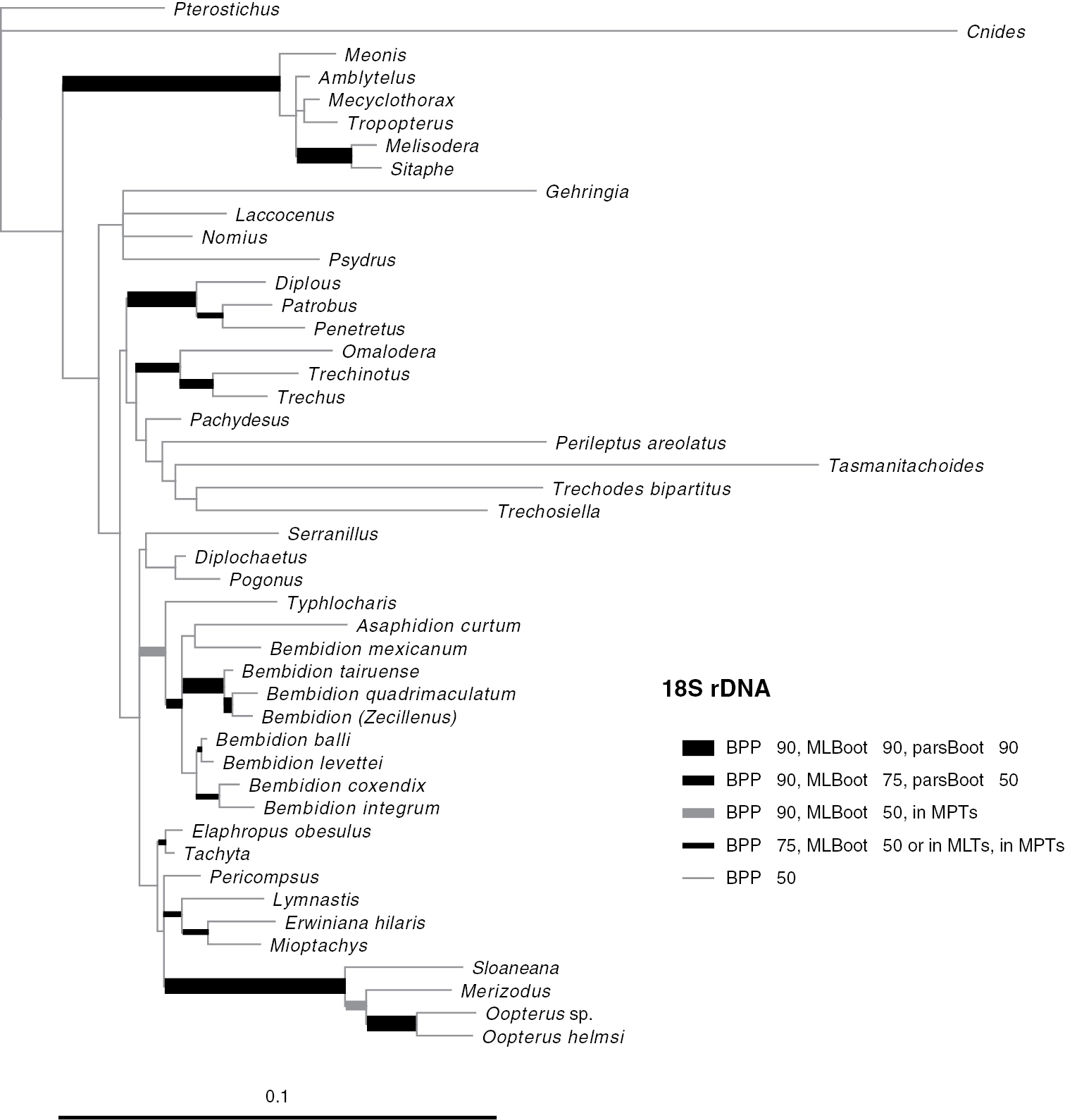
Majority-rule consensus tree of trees sampled in Bayesian analysis, with branch lengths proportional to average branch lengths across trees that contain that branch, for 18S rDNA data. See caption of Fig. 2 for additional details.
Majority-rule consensus tree of trees sampled in Bayesian analysis, with branch lengths proportional to average branch lengths across trees that contain that branch, for 18S rDNA data. See caption of Fig. 2 for additional details.
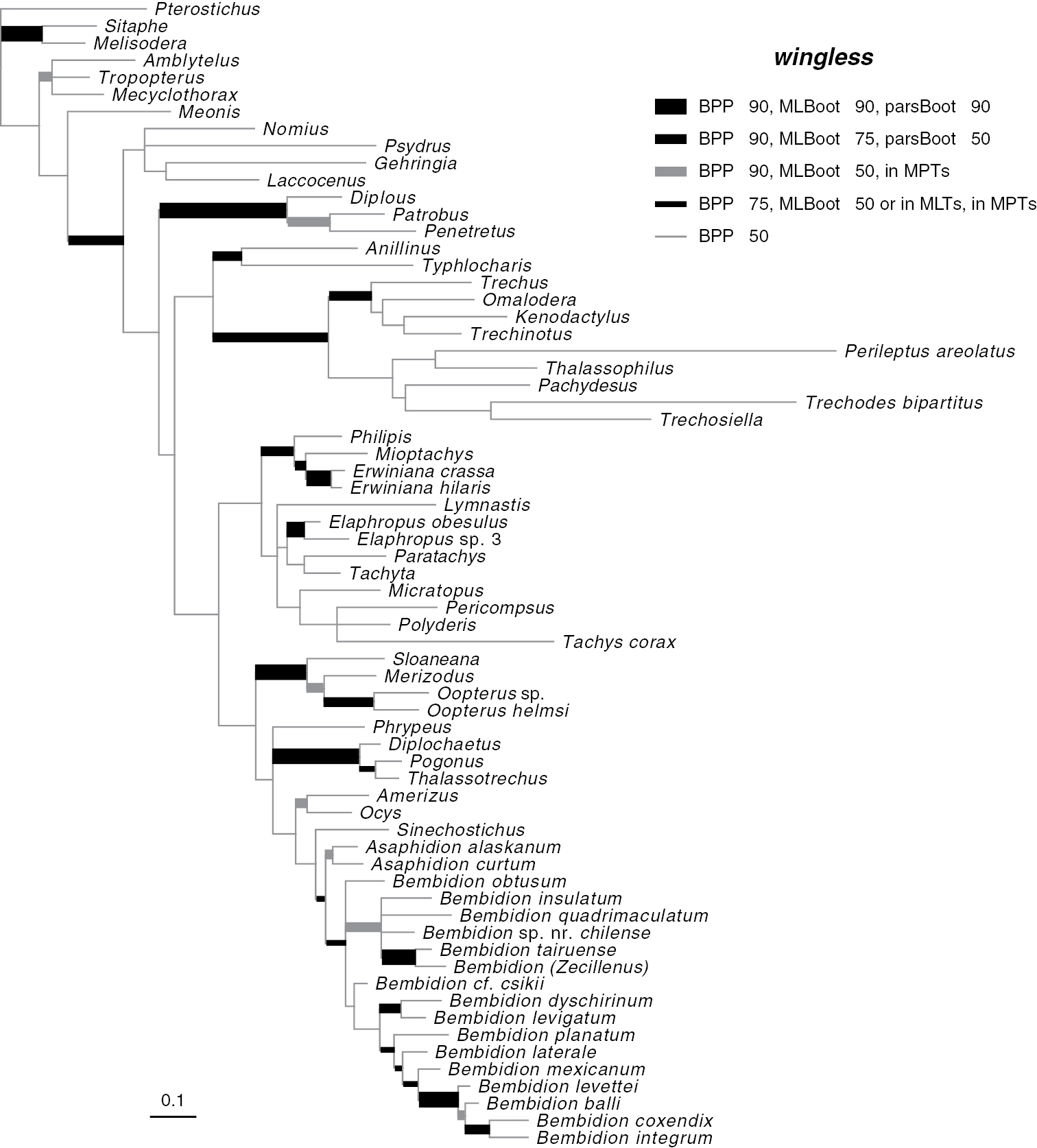
Majority-rule consensus tree of trees sampled in Bayesian analysis, with branch lengths proportional to average branch lengths across trees that contain that branch, for the complete wingless data. See caption of Fig. 2 for additional details.
Majority-rule consensus tree of trees sampled in Bayesian analysis, with branch lengths proportional to average branch lengths across trees that contain that branch, for the complete wingless data. See caption of Fig. 2 for additional details.
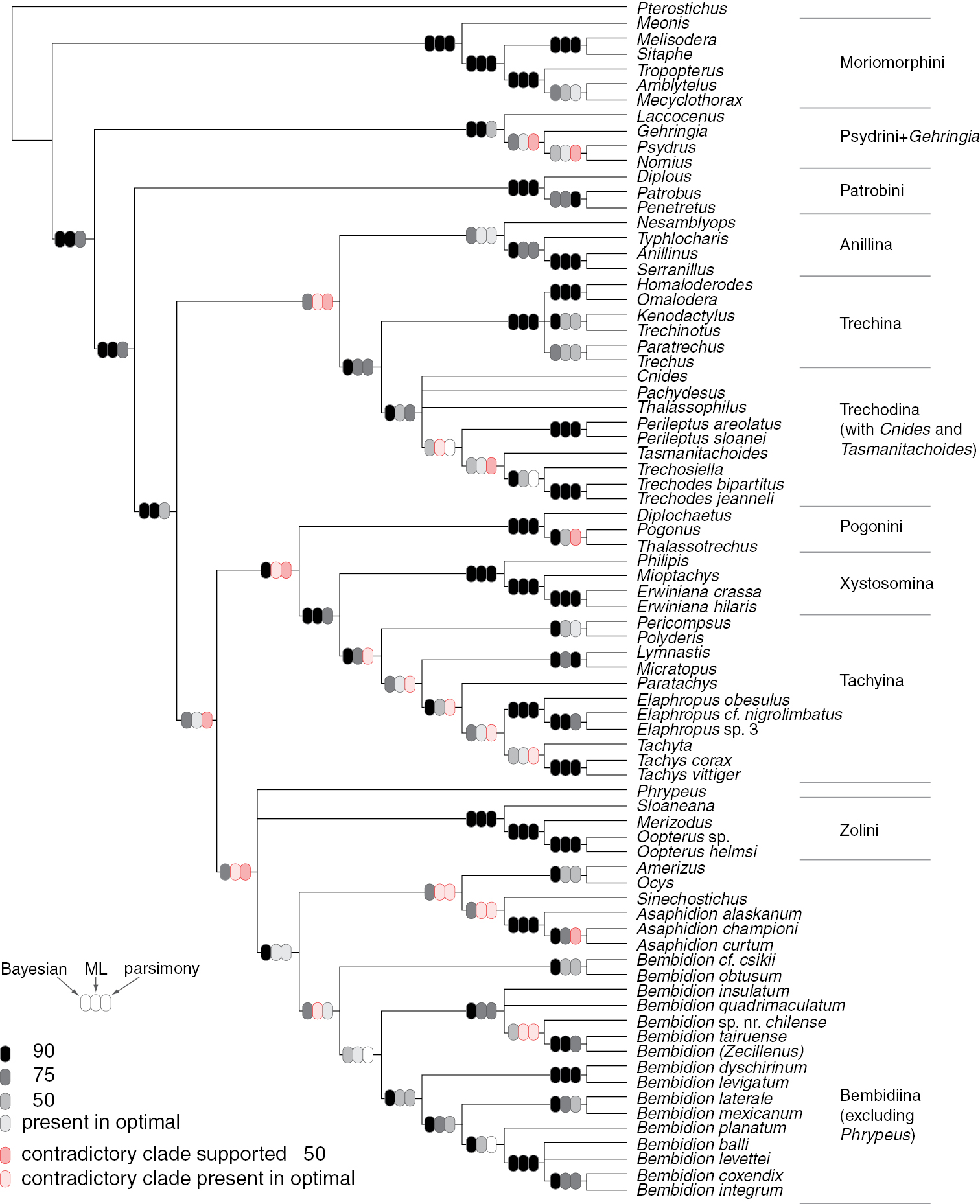
Majority-rule consensus tree of trees sampled in Bayesian analysis for all three genes analyzed together. Ovals on branches indicate support for the clade based upon Bayesian (left), maximum likelihood (center), and parsimony (right) analyses. Darkest tones indicate strongest support for (grays and black) or against (pinks) the clade, with values indicating posterior probability expressed as a percentage (Bayesian), or bootstrap percentage (likelihood and parsimony).
Majority-rule consensus tree of trees sampled in Bayesian analysis for all three genes analyzed together. Ovals on branches indicate support for the clade based upon Bayesian (left), maximum likelihood (center), and parsimony (right) analyses. Darkest tones indicate strongest support for (grays and black) or against (pinks) the clade, with values indicating posterior probability expressed as a percentage (Bayesian), or bootstrap percentage (likelihood and parsimony).
Support values for various groups outside of Bembidiini (sensu lat.): B: Bayesian posterior probability, expressed as a percentage; ML: Maximum likelihood analysis; P: parsimony analysis. For maximum likelihood and parsimony analyses, numbers indicate the bootstrap support expressed as a percentage; check marks indicate that the clade is present in the optimal (maximum likelihood or post parsimonious) trees but with bootstrap value below 50; x indicates that a contradictory clade was present in the optimal (maximum likelihood or post parsimonious) trees but with bootstrap value below 50; negative values indicate Bayesian posterior probability or bootstrap support for a contradictory clade. Boxes in gray to black indicate support for the clade; boxes in pink to red indicate support against that clade, with darker colors indicating stronger support. Dashes indicate no support for or against the clade because of insufficient taxon sampling for that gene; blank boxes indicate no support for or against the clade because of lack of resolution in the inferred trees. Abbreviations: “inc.” = “including”, “exc.” = “excluding”.
| merged | 28S rDNA | 18S rDNA | wg, all nucleotides | wg, well-aligned nucleotides | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | ML | P | B | ML | P | B | ML | P | B | ML | P | B | ML | P | |
| Moriomorphini | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | -100 | -78 | -75 | x | ✓ | |
| Psydrini+ Gehringia | 100 | 99 | 70 | 100 | 99 | 70 | 56 | x | x | 88 | ✓ | 88 | ✓ | ✓ | |
| Patrobini+ Trechitae | 100 | 92 | 80 | 54 | 58 | 66 | -66 | x | x | 100 | 58 | 100 | 59 | ✓ | |
| Trechitae | 100 | 93 | 66 | 100 | 70 | 53 | -66 | x | x | 100 | 77 | 100 | 75 | ✓ | |
| Trechini | 99 | 79 | 86 | 100 | 83 | 64 | -66 | x | x | 100 | 98 | 78 | 100 | 98 | 75 |
| Trechina | 100 | 95 | 98 | 62 | 70 | 96 | 100 | 80 | 70 | 100 | 99 | 80 | 100 | 100 | 79 |
| Trechodina | 94 | 64 | 78 | -66 | x | ✓ | -89 | x | x | 100 | 82 | 100 | 79 | ||
| Tasman. with Trechini | 99 | 79 | 86 | 100 | 83 | 64 | 95 | ✓ | 60 | - | - | - | - | - | - |
| Pogonini | 100 | 100 | 100 | 100 | 98 | 97 | 100 | 72 | 72 | 100 | 100 | 100 | 100 | 100 | 100 |
| Zolini | 100 | 100 | 100 | 100 | 96 | 96 | 100 | 100 | 100 | 100 | 99 | 97 | 100 | 97 | 92 |
| merged | 28S rDNA | 18S rDNA | wg, all nucleotides | wg, well-aligned nucleotides | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | ML | P | B | ML | P | B | ML | P | B | ML | P | B | ML | P | |
| Moriomorphini | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | -100 | -78 | -75 | x | ✓ | |
| Psydrini+ Gehringia | 100 | 99 | 70 | 100 | 99 | 70 | 56 | x | x | 88 | ✓ | 88 | ✓ | ✓ | |
| Patrobini+ Trechitae | 100 | 92 | 80 | 54 | 58 | 66 | -66 | x | x | 100 | 58 | 100 | 59 | ✓ | |
| Trechitae | 100 | 93 | 66 | 100 | 70 | 53 | -66 | x | x | 100 | 77 | 100 | 75 | ✓ | |
| Trechini | 99 | 79 | 86 | 100 | 83 | 64 | -66 | x | x | 100 | 98 | 78 | 100 | 98 | 75 |
| Trechina | 100 | 95 | 98 | 62 | 70 | 96 | 100 | 80 | 70 | 100 | 99 | 80 | 100 | 100 | 79 |
| Trechodina | 94 | 64 | 78 | -66 | x | ✓ | -89 | x | x | 100 | 82 | 100 | 79 | ||
| Tasman. with Trechini | 99 | 79 | 86 | 100 | 83 | 64 | 95 | ✓ | 60 | - | - | - | - | - | - |
| Pogonini | 100 | 100 | 100 | 100 | 98 | 97 | 100 | 72 | 72 | 100 | 100 | 100 | 100 | 100 | 100 |
| Zolini | 100 | 100 | 100 | 100 | 96 | 96 | 100 | 100 | 100 | 100 | 99 | 97 | 100 | 97 | 92 |
Support values for various groups of Bembidiini (sens. lat.). See legend of Table 4 for more explanation.
| merged | 28S rDNA | 18S rDNA | wg, all nucleotides | wg, well-aligned nucleotides | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | ML | P | B | ML | P | B | ML | P | B | ML | P | B | ML | P | |
| Bembidiini (sens. lat.) | -88 | x | x | -85 | x | x | -98 | x | x | -100 | -63 | x | -100 | -56 | x |
| Tachyina+ Xystosomina | 100 | 92 | 85 | 100 | 72 | 66 | -62 | x | x | 68 | x | x | 80 | ✓ | ✓ |
| Tachyina | 100 | 88 | x | 99 | 67 | x | -62 | x | x | 99 | ✓ | x | 87 | ✓ | x |
| Xystosomina | 100 | 99 | 100 | 100 | 80 | 75 | 89 | 71 | 69 | 99 | 88 | 85 | 100 | 88 | 89 |
| Anillina (inc. Nesamblyops) | 87 | ✓ | ✓ | 93 | ✓ | ✓ | - | - | - | - | - | - | - | - | - |
| Bembidiina inc. Phrypeus | x | x | x | x | - | - | - | - | x | 51 | x | x | |||
| Bembidiina exc. Phrypeus | 91 | ✓ | ✓ | x | x | 100 | 76 | 78 | 100 | ✓ | ✓ | 89 | x | x | |
| Bembidion (sens. lat.) | 75 | x | ✓ | x | -54 | x | -57 | 89 | ✓ | ✓ | 83 | x | |||
| Zecillenus in Bembidion | 100 | 91 | 89 | 91 | ✓ | ✓ | 100 | 98 | 98 | 100 | 98 | 96 | 100 | 95 | 93 |
| Bembidion taiurense + Zecillenus | 100 | 91 | 89 | 52 | ✓ | ✓ | -100 | -91 | -91 | 100 | 98 | 96 | 100 | 95 | 93 |
| Bembidion series | 100 | 87 | 88 | 91 | ✓ | ✓ | 100 | 98 | 98 | 98 | 60 | 67 | 97 | 57 | 66 |
| Cillenus in Bembidion | 100 | 84 | 64 | 99 | 79 | 78 | - | - | - | 99 | ✓ | ✓ | 93 | ✓ | ✓ |
| merged | 28S rDNA | 18S rDNA | wg, all nucleotides | wg, well-aligned nucleotides | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | ML | P | B | ML | P | B | ML | P | B | ML | P | B | ML | P | |
| Bembidiini (sens. lat.) | -88 | x | x | -85 | x | x | -98 | x | x | -100 | -63 | x | -100 | -56 | x |
| Tachyina+ Xystosomina | 100 | 92 | 85 | 100 | 72 | 66 | -62 | x | x | 68 | x | x | 80 | ✓ | ✓ |
| Tachyina | 100 | 88 | x | 99 | 67 | x | -62 | x | x | 99 | ✓ | x | 87 | ✓ | x |
| Xystosomina | 100 | 99 | 100 | 100 | 80 | 75 | 89 | 71 | 69 | 99 | 88 | 85 | 100 | 88 | 89 |
| Anillina (inc. Nesamblyops) | 87 | ✓ | ✓ | 93 | ✓ | ✓ | - | - | - | - | - | - | - | - | - |
| Bembidiina inc. Phrypeus | x | x | x | x | - | - | - | - | x | 51 | x | x | |||
| Bembidiina exc. Phrypeus | 91 | ✓ | ✓ | x | x | 100 | 76 | 78 | 100 | ✓ | ✓ | 89 | x | x | |
| Bembidion (sens. lat.) | 75 | x | ✓ | x | -54 | x | -57 | 89 | ✓ | ✓ | 83 | x | |||
| Zecillenus in Bembidion | 100 | 91 | 89 | 91 | ✓ | ✓ | 100 | 98 | 98 | 100 | 98 | 96 | 100 | 95 | 93 |
| Bembidion taiurense + Zecillenus | 100 | 91 | 89 | 52 | ✓ | ✓ | -100 | -91 | -91 | 100 | 98 | 96 | 100 | 95 | 93 |
| Bembidion series | 100 | 87 | 88 | 91 | ✓ | ✓ | 100 | 98 | 98 | 98 | 60 | 67 | 97 | 57 | 66 |
| Cillenus in Bembidion | 100 | 84 | 64 | 99 | 79 | 78 | - | - | - | 99 | ✓ | ✓ | 93 | ✓ | ✓ |
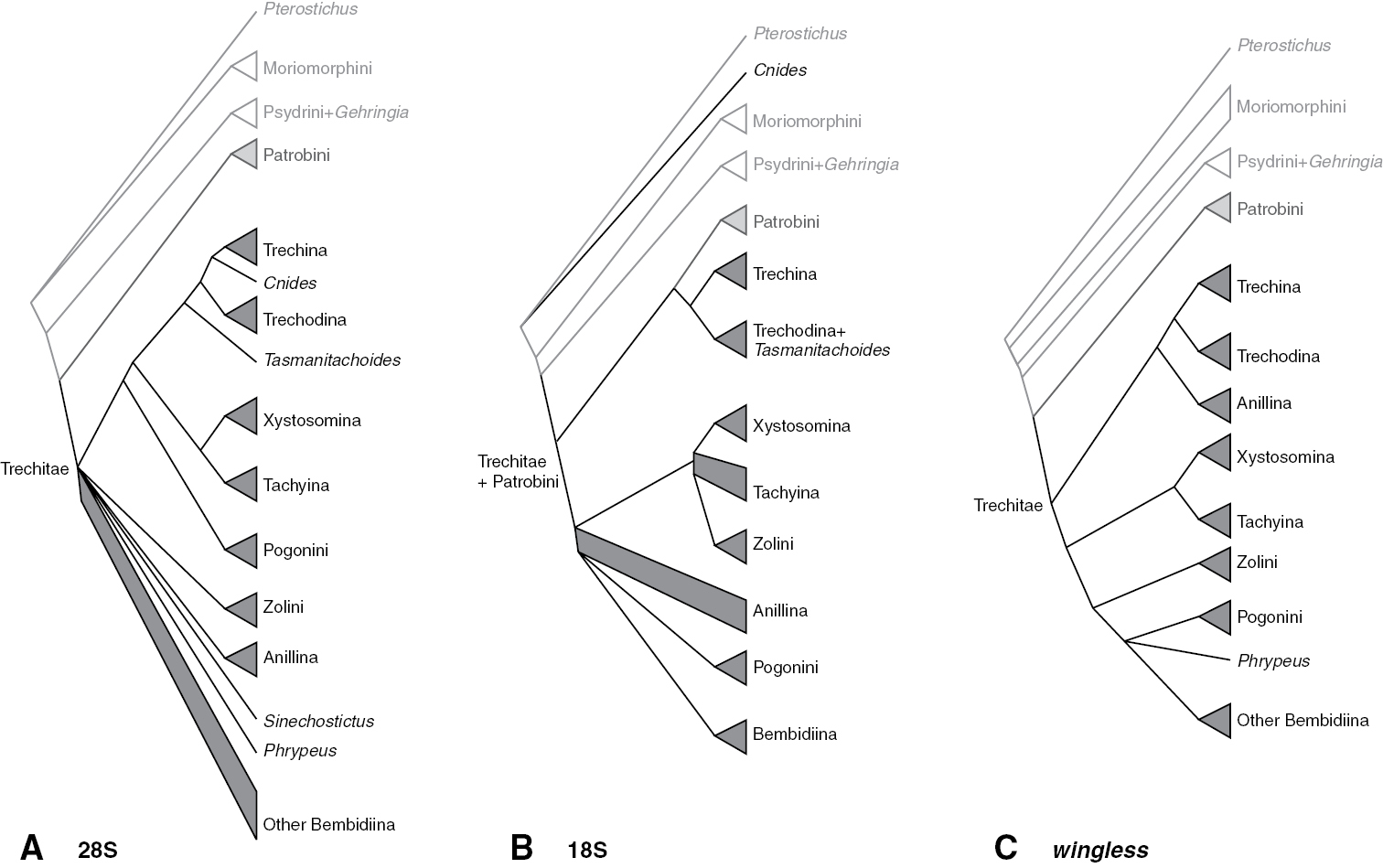
Summary of subtribal and tribal relationships supported by individual genes. Triangles indicate monophyletic groups; quadrangles represent paraphyletic groups A 28S rDNA B 18S rDNA C wingless.
Summary of subtribal and tribal relationships supported by individual genes. Triangles indicate monophyletic groups; quadrangles represent paraphyletic groups A 28S rDNA B 18S rDNA C wingless.
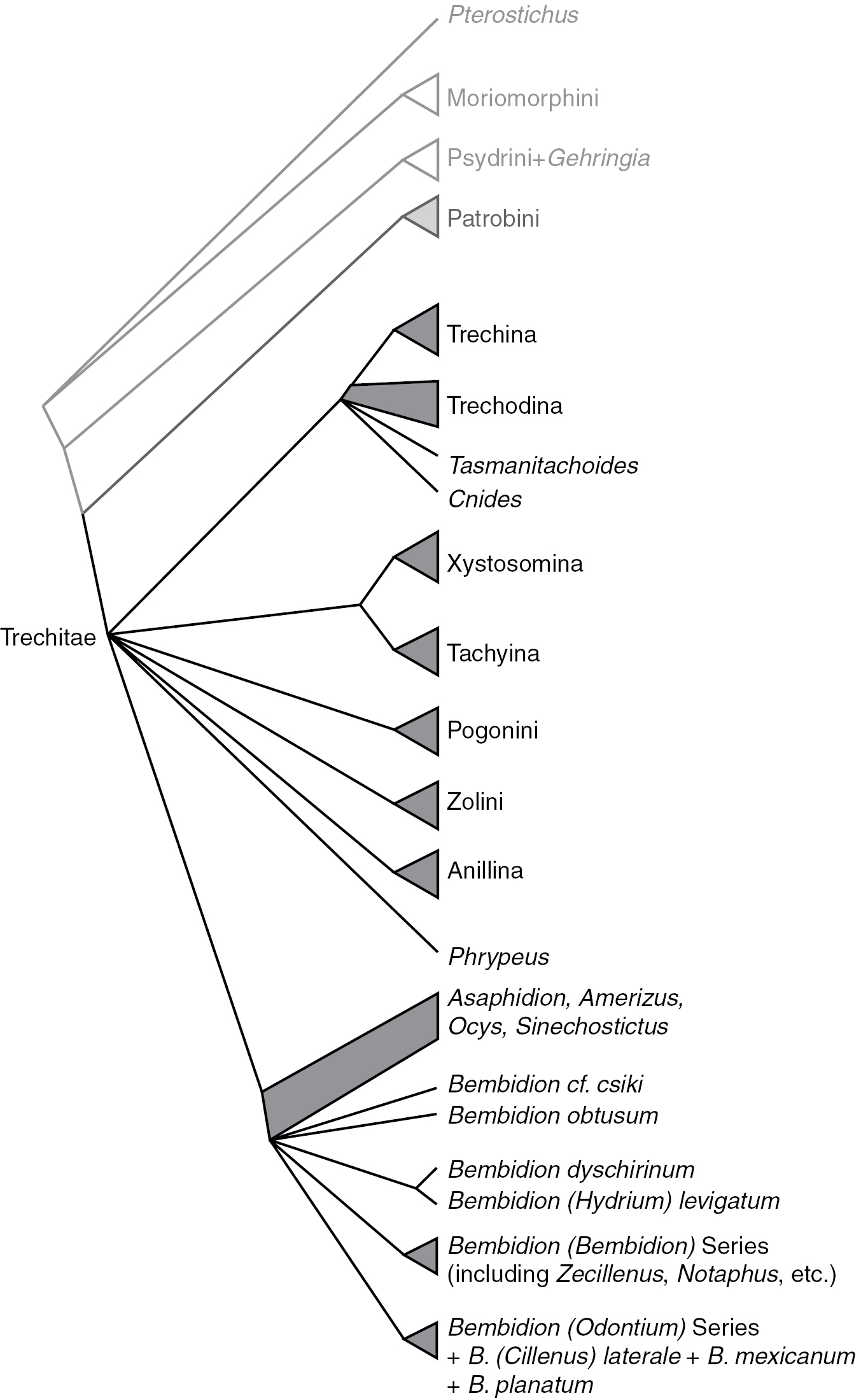
Summary of relationships in Trechitae and related taxa. Branches (including those subtended by triangles) indicate monophyletic groups supported by the combined analyses and at least two of the genes; quadrangles indicate groups whose status is unresolved.
Summary of relationships in Trechitae and related taxa. Branches (including those subtended by triangles) indicate monophyletic groups supported by the combined analyses and at least two of the genes; quadrangles indicate groups whose status is unresolved.
Support values for various groups in analyses of 18S constrained to keep Trechitae or Trechini monophyletic are shown in Table 6.
Results for the reanalysis of Grebennikov’s (2008) larval data are presented in Fig. 1B, and described in more detail in the text, below.
Support values for various groups outside of Bembidiini (sensu lat.): comparison of results for 18S rDNA between unconstrained and constrained analyses. For the “Trechitae constrained” analyses, all of Trechitae was constrained to be monophyletic. For the “Trechini constrained analyses”, all of Trechini was constrained to be monophyletic for the Bayesian analysis; for the remaining analyses, Trechini was constrained to be monophyletic, except that Tasmanitachoides was unconstrained, and could move anywhere in the tree if that were optimal. “n/a” indicates that support values for that group are irrelevant as the group was forced to be monophyletic; for meaning of other symbols and colors, see legend of Table 4.
| No Constraints | Trechitae constrained | Trechini constrained | |||||||
|---|---|---|---|---|---|---|---|---|---|
| B | ML | P | B | ML | P | B | ML | P | |
| Psydrini+ Gehringia | 56 | x | x | 59 | x | -63 | 50 | ✓ | x |
| Patrobini+ Trechitae | -66 | x | x | 99 | 95 | 94 | 94 | ✓ | 53 |
| Trechitae | -66 | x | x | n/a | n/a | n/a | x | ✓ | |
| Trechini | -66 | x | x | -85 | x | ✓ | n/a | n/a | n/a |
| Trechina | 100 | 80 | 70 | 99 | 82 | 69 | 99 | 82 | 70 |
| Trechodina | -89 | x | x | -85 | x | 64 | 3 | x | |
| Tasman. with Trechini | 95 | ✓ | 60 | 89 | ✓ | 67 | 100 | 76 | 84 |
| No Constraints | Trechitae constrained | Trechini constrained | |||||||
|---|---|---|---|---|---|---|---|---|---|
| B | ML | P | B | ML | P | B | ML | P | |
| Psydrini+ Gehringia | 56 | x | x | 59 | x | -63 | 50 | ✓ | x |
| Patrobini+ Trechitae | -66 | x | x | 99 | 95 | 94 | 94 | ✓ | 53 |
| Trechitae | -66 | x | x | n/a | n/a | n/a | x | ✓ | |
| Trechini | -66 | x | x | -85 | x | ✓ | n/a | n/a | n/a |
| Trechina | 100 | 80 | 70 | 99 | 82 | 69 | 99 | 82 | 70 |
| Trechodina | -89 | x | x | -85 | x | 64 | 3 | x | |
| Tasman. with Trechini | 95 | ✓ | 60 | 89 | ✓ | 67 | 100 | 76 | 84 |
We here discuss in turn the evidence available for various relationships within trechites. As significant results have been found within the outgroups sampled, we will discuss these first.
Outgroup structure: Monophyly of MoriomorphiniAmong the carabids currently considered to belong to Psydrini (sens. lat.), only three genera belong to Psydrina in the strict sense: Psydrus (North America), Nomius (Holarctic and Africa), and Laccocenus (Australia). The remaining genera are arrayed in multiple subtribes (Baehr 1998; Moore 1963), and are restricted to the Southern Hemisphere, primarily in temperate areas, except for members of the genus Mecyclothorax, which occur as far north as Hawai’i (Liebherr 2006; 2008; 2009). Sequences of 18S rDNA indicate that all psydrines other than Psydrus, Nomius, and Laccocenus belong to a clade, termed the “austral psydrines” by Maddison et al. (1999a). Three apomorphies of adult structure (Baehr 1999) also indicate monophyly of the austral psydrines. In addition, 18S rDNA indicated that austral psydrines were not closely related to psydrines in the strict sense (Maddison, et al. 1999a).
Our data indicate that Psydrina are not closely related to austral psydrines. While a strong test of this hypothesis with 28S and wingless would require more extensive sampling of non-trechites than we have done, all three genes we studied suggest that Psydrini in the classical sense, containing Psydrus, Nomius, Laccocenus, and the austral psydrines, is not monophyletic (Figs 2–5). Combined with evidence provided by the more extensive 18S rDNA taxon sampling of Maddison et al. (1999a), and the morphological data (Baehr 1999), these two groups should be in separate taxa. We therefore remove austral psydrines from Psydrini, and place them in their own tribe. The valid name for this tribe is Moriomorphini Sloane (1890: 646).
Our results indicate strong support for monophyly of Moriomorphini from both 18S rDNA and 28S rDNA (Table 4). Most analyses of the wingless gene suggest instead that the moriomorphines form a grade, although parsimony analysis of the well-aligned nucleotides does support monophyly of the group. More extensive sampling of wingless sequences of non-trechites is needed to examine this further.
Outgroup structure: relationship of GehringiiniGehringiini are a small group (five known species; Baehr et al. 2009) of minute carabids of uncertain relationships. Morphological data places them variously as sister group of Paussinae (R.T. Bell at the 1983 Entomological Society of America meetings; Beutel 1992), with Trachypachidae (Kryzhanovskij 1976; Lindroth 1969), among the basal carabids (Bell 1967; Darlington 1933; Jeannel 1941), or a member of Trechitae or a relative of Psydrini (Bell 1967; Darlington 1933; Erwin 1984; 1985; Hammond 1979). Sequences of 18S rDNA suggested a possible placement near Cymbionotum (Maddison et al. 1999a). As most of the groups that have been proposed as near-relatives of gehringiines are not included in the current study, we cannot conduct a definitive test. However, it is remarkable that separate analyses of each of the three genes, plus the combined analyses, indicate at least some level of support for having Gehringia belonging to a clade with the true Psydrini (Figs 2–5, Table 4), echoing the placement “as a basal psydrite group” by Erwin (1985).
Relationships of Patrobini to TrechitaeTrechitae + Patrobini share a number of synapomorphies in adult structure (Zamotajlov 2002), larval characteristics (Arndt 1993; Arndt 1998; Bousquet and Grebennikov 1999; Houston and Luff 1975; Müller 1975; Zamotajlov 2002), and female abdominal structure (Deuve 1993) that suggest they form a clade. This result was corroborated by 18S rDNA (Maddison et al. 1999a). In contrast, Roig-Juñent and Cicchino (2001) has the morimorphine subgroup Amblytelini as sister to Patrobini, which are together sister to Trechitae.
Consistent with most morphological data, our 28S and wingless data support monophyly of Patrobini plus Trechitae (Table 5), as does 18S if Trechini is constrained to be monophyletic (Table 6).
Monophyly of TrechitaeShared derived characters that provide evidence for monophyly of Trechitae are found in multiple character systems. Protarsomeres of adult males are uniquely dentate and dilated on the mesal side (Roig-Juñent and Cicchino 2001). There are derived traits of larval structure (Arndt 1993; Grebennikov and Maddison 2000; 2005). Males lack chiasmata in meiosis (Serrano 1981), although this has been examined in relatively few genera, and in no zolines. Males of almost all other carabids, including patrobines, are chiasmatic (Galián et al. 1994; Serrano and Galian 1998), with the possible exception of isolated separate origins within the distantly-related Carabini (Yadav and Burra 1987) and Harpalini (Serrano 1981). The lack of chiasmata in trechites is thus a notable synapomorphy. 18S rDNA has also provided evidence of monophyly (Maddison, et al. 1999a).
Trechitae is strongly supported as monophyletic in 28S, wingless, and the merged matrix (Table 5). In contrast, because of the placement of Cnides outside of Trechitae (Fig. 3), as discussed in the next section, 18S provides evidence against the monophyly of Trechitae. If Cnides is forced to stay within Trechini, however, 18S provides no clear signal for or against monophyly (Table 6).
Monophyly and phylogeny of TrechiniThe primary synapomorphy suggesting the monophyly of Trechini is the presence of deep furrows on the dorsal surface of the head (Jeannel 1926). In its extreme form, this state is unique within carabids, but there are also trechines with relatively shallow, less extensive furrows that are not dissimilar to those found in other carabids. There are, however, several derived states in larval characters that indicate monophyly of Trechini (Grebennikov 2008; Grebennikov and Maddison 2005).
Our results from 28S, wingless, and the merged matrices indicate strong support for monophyly of Trechini (Table 4), if Tasmanitachoides is included within the tribe (as discussed in the next section). In contrast, 18S provides moderate evidence against the monophyly of trechines, because of the placement of Cnides outside of Trechitae (Fig. 3). However, the exceedingly divergent Cnides 18S sequence (note the length of its branch in Fig. 3) makes artificial attraction of the long branch (Felsenstein 1978) to distantly related outgroups a reasonable explanation.
In Jeannel’s great work (1926; 1927; 1928; 1930) trechines are divided into five groups. One of most distinctive groups are the trechodines, a predominately Southern-Hemisphere group, characterized by a basal bulb of the male aedeagus that is open dorsally, as opposed to the closed basal bulb found in other trechines. Among the taxa we have sequenced, trechodines are represented by Pachydesus, Thalassophilus, Cnides, and Trechodes. Jeannel’s other four groups are each represented in our data: his aepines by Kenodactylus, his homaloderines by Omalodera, Homaloderodes, and Trechinotus, his perileptines by Perileptus, and with Paratrechus and Trechus representing his trechines in the strict sense.
Trechini is a diverse group, with complex patterns of morphological variation, and has been subject to many different classification schemes. For example, some classifications view homaloderines as members of Trechina proper (e.g., Casale and Laneyrie 1982; Lorenz 2005). All classifications consider trechodines to be a distinct group, although the placement of Perileptus has varied. Jeannel (1926; 1927; 1928; 1930), Casale and Laneyrie (1982), and Lorenz (2005) treated perileptines as a group distinct from trechodines. Uéno (1989), however, considered Perileptus and related genera to be a trechodines, citing a misunderstanding by other authors of the structure of the male aedeagus in Perileptus. None of these classifications are based upon explict phylogenetic analyses, however.
In recent years larval structure and DNA sequences have been used in a few explicit phylogenetic studies within trechines. The most complete available larval data (Grebennikov 2008; Grebennikov and Maddison 2005), suggests that neither trechodines nor trechines are monophyletic, with trechodines forming a grade within Trechina. However, this result is not robust to the alternative assumptions employed by Grebennikov (2008). We have reanalyzed his data using his initial ordering assumptions, and find that relationships within trechines are more ambiguous than those shown in Grebennikov’s (2008) Fig. 3 (our Fig. 1B). Notably, some most-parsimonious trees have Trechodina and Trechina each monophyletic, and sister to each other (not shown). The only other paper to explicitly examine the phylogeny of trechines using modern analytical methods is that of Faille et al. (2010), which used 28S, 18S, and mitochondrial genes to infer the phylogeny of some European trechines, a very different question, with different taxon sampling, from the question of worldwide relationships examined here.
Our results (Figs 2–5, Table 4) confirm or refute several previous proposals. Trechina (including Jeannel’s homaloderines and the aepiine Kenodactylus) is monophyletic, supported by all three genes individually, and by analyses of the combined data. Perileptus is a trechodine, as supported by all three genes, and by the combined data, as predicted by Uéno (1989). There is weak evidence that trechodines are monophyletic, with wingless and the merged data in support, 28S ambiguous, and 18S speaking against monophyly.
Relationships of TasmanitachoidesIn the original description of Tasmanitachoides, Erwin (1972) considered the genus to be “an early off-shoot of the tachyine lineage which gave rise to the Anillina”. He notes that they “show similarities to the trechines”, but view those similarities as symplesiomorphies. The genus's placement in trechites has been examined in detail only once since then, in Grebennikov’s (2008) paper on larval characters. As Grebennikov reports, our DNA data indicates (as does his larval data) that Tasmanitachoides is not a tachyine, but instead shows affinity to trechines (Figs 2, 3, 5; Table 4). In our data, this relationship is supported by both ribosomal genes; we did not manage to acquire wingless sequence from Tasmanitachoides, and so that gene is at the moment mute on relationships of the genus.
Monophyly of ZoliniLiebherr and Will’s (1999) study of female genitalic characters suggested that zolines were not monophyletic, with Oopterus and Merizodus appearing separately on their inferred phylogeny. However, as they note, the number of characters used was small enough to confer limited confidence in that result. Roig-Juñent and Chicchino’s (2001) study of adult structure, which included members of all subtribes of zolines, weakly supported monophyly of the group. 18S rDNA strongly supports monophyly of Oopterus + Merizodus + Sloaneana (Maddison, et al. 1999a; note that in that paper, Oopterus helmsi is referred to as “Zolus helmsi”).
Although our study cannot be a strong test of the monophyly of zolines, as we do not have members of two subtribes (Sinozolina and Chalteniina), the three genera we have examined (Oopterus, Merizodus, and Sloaneana) are strongly supported as a clade, in all three genes and in all analyses (Table 4).
Monophyly of PogoniniPogonini is a tribe of about 70 species, most of which live in saline habitats (Bousquet and Laplante 1997). Except for the recently described genus, Olegius Komarov (1996), the monophyly of the tribe is not in question. Grebennikov and Bousquet (1999) found three synapomorphies in larvae that suggest monophyly of the tribe. Our results confirm this, with all genes and all analyses indicating that Pogonus + Diplochaetus + Thalassotrechus form a clade (Table 4).
Monophyly of BembidiiniAs delimited in this paper, Bembidiini includes four subtribes: Bembidiina, a large group of over 1, 200 species primarily found in temperate regions, and which includes the larger bembidiines; Tachyina, the second large group, mostly ground-dwelling, centered in warmer regions; Xystosomina, a primarily arboreal group most abundant in the Neotropics; and Anillina, containing very small, often blind, mostly soil-dwelling carabids. In some classifications, these groups are treated as separate tribes, in part as there is only one derived character that suggests that the group is monophyletic (the small terminal article of the maxillary and labial palps of adults, a character that occurs in some trechines, e.g., Perileptus), and in part as the subtribes are relatively uniform within themselves, but with several characters that distinguish them one from another. An analysis of larval characters (Grebennikov and Maddison 2005) gives no indication of monophyly of the tribe.
Our results are consistent with the view that Bembidiini is a heterogeneous group, with all three genes and the combined analysis indicating non-monophyly (Table 5), although with no consistent pattern of particular subgroups of Bembidiini being related to non-bembidiines. More details are provided under the discussions of each subtribe, below.
Monophyly of XystosominaThis subtribe was established by Erwin (1994) for six New World genera (four of which have arboreal members some species of which also use leaf-litter, and two having subcortical members) and one arboreal genus, Philipis, from tropical Australia. While no explicit phylogenetic analysis supporting monophyly of this group has been published, our data (which includes three of the more divergent genera) supports monophyly, with all three genes and the combined analyses indicating that xystosomines form a clade (Table 5).
Monophyly of TachyinaMonophyly of Tachyina exclusive of Xystosomina is supported by the obliquely notched front tibia of adults (Erwin 1994), a state apparently independently derived in anillines (as discussed below). This result is not consistent with larval data, which suggests that Tachyta (a tachyine) is more closely related to Mioptachys (a xystosomine) than it is to other tachyines (Grebennikov and Maddison 2005).
Bayesian analysis of 28S, wingless, and the merged matrix supports the monophyly of Tachyina (Table 5). However, this is result is not supported by Bayesian analysis of 18S rDNA, and parsimony analyses of all genes speak against monophyly of tachyines. A denser taxon sampling of both tachyines and xystosomines is needed to resolve this conflict.
Monophyly and origin of AnillinaMost anillines are minute, blind, wingless, pale inhabitants of soil and deep leaf litter; members of a few genera have small eyes, e.g., Nesamblyops from New Zealand (Moore 1980) and Microdipnodes from Africa (Jeannel 1963). As most of the distinctive characteristics of anillines are expected to evolve in small beetles that live in soil, it is possible that anillines represent a grade that has repeatedly and independently evolved from above-ground trechites as those lineages went underground (Erwin 1982). Erwin proposed in particular that anillines may represent “a grade of numerous parallel lineages derived from Paratachys and allies.”
If Erwin (1982) is right that polyphyletic origin of anillines explains how these presumably slowly-dispersing beetles would be present on multiple continents and remote islands, then our sample of anillines from North America, Europe, and New Zealand should be a good test of his hypothesis. The only gene for which we have data from all four sampled genera is 28S. These data refute Erwin’s hypothesis, as the anillines are strongly supported as monophyletic (Fig. 2 and Table 4).
The exclusion of anillines from the Tachyina + Xystosomina clade (Fig. 7) suggests that the obliquely notched anterior tibia in anillines and Tachyina (Erwin 1982) arose independently in the two groups.
Monophyly of BembidiinaBembidiina comprises all Bembidiini that do not belong to the other tribes; the group is defined by the lack of derived characters of its members. As such, evidence for monophyly is not evident in morphological data (Grebennikov 2008; Grebennikov and Maddison 2005). Our data show very limited evidence of monophyly of Bembidiina (only the Bayesian analysis of well-aligned nucleotides of wingless gives slight support) and evidence against monophyly from other analyses and from 28S rDNA. However, Bembidiina excluding Phrypeus is supported as monophyletic by 18S rDNA, wingless, and the merged matrix (Table 5). Further investigations of Phrypeus need to be conducted to see if it should be excluded from Bembidiina.
Relationships within BembidiinaThe majority of species within subtribe Bembidiina belong to the genus Bembidion. Bembidion was regarded by Carl Lindroth (1963; 1980) and (as a result) most North American carabidologists as a very large genus comprising all non-tachyine, non-xystosomine, non-anilline bembidiines that possess a distinctive brush in the internal sac of the male genitalia, and with male foretarsomeres with adhesive setae arranged in a rows. Three groups of Bembidiina without a brush (Phrypeus, Zecillenus, Bembidarenas Erwin) have been excluded by Lindroth and from Bembidion on this basis, although the same was not done for some South American species also lacking a brush (e.g., members of the subgenus Antiperyphanes), which were still maintained within Bembidion. A similar brush is present in two groups outside Bembidion (in this classification): Asaphidion and some Xystosomina (Erwin 1994). The brush in xystosomines is likely convergent, as they share apomorphies that place them with a group that lacks a brush, the tachyines. Asaphidion, while having the brush, has distinctive dorsal texture and is unique within Trechitae of having adhesive setae on the foretarsomere of males arranged randomly, not in rows (Maddison, 1993). However, these traits are likely autapomorphies of Asaphidion, and thus not significant as evidence of relationships to other groups.
More recently, other groups have been excluded from Bembidion in most classifications, including Ocys, Cillenus, Amerizus, Sinechostictus, Orzolina Machado, and Caecidium Uéno. Thus, current classifications have a very large, poorly-defined genus Bembidion surrounded by a number of small “satellite” genera that are each defined in good part based upon autapomorphies. Bembidion in either the traditional or modern senses has no known synapomorphies of its members, and thus it would not be surprising if some of these satellite genera were found to be derived lineages within Bembidion. However, as there have been no comprehensive phylogenetic analyses at this level within Bembidiina, the lack of known derived states does not indicate that Bembidion as currently defined is non-monophyletic. Only some recent papers on larval characters (Grebennikov 2008; Grebennikov and Maddison 2005) employ cladistic analyses or numerical analyses of character matrices, and they do not have dense-enough taxon sampling to address most of these issues. Thus, there is little existing published evidence for or against the monophyly of Bembidion or other major subgroups within Bembidiina.
Our data suggest that some of these smaller genera are indeed outside of Bembidion. The distant relationship of Phrypeus to Bembidion is discussed above. Both Amerizus and Ocys fall outside of Bembidion in the broad sense in analyses of 28S rDNA, wingless, and the merged matrix (Figs 2, 4, 5). The placement of Sinechostictus has varied through time. These beetles very closely resemble in general habitus members of the Ocydromus complex of Bembidion, with which they have been placed by several authors (e.g., Antoine 1955; Jeannel 1941). In contrast, Perrault (1981) and Ortuño and Toribio (2005) considered Sinechostictus to be a group distinct from Bembidion based on spermathecal and aedeagal structures. Grebennikov (1997) found that Sinechostictus larvae lack some synapomorphies of Bembidion + Asaphidion, a result supported by further larval studies (Grebennikov 2008; Grebennikov and Maddison 2005). Our results corroborate this result, with wingless (Fig. 4) and the merged data (Fig. 5) suggesting that Sinechostictus falls outside of Bembidion.
On the other hand, some of the groups that have been recently considered outside of Bembidion are evidently derived members of that genus.
Hydrium, considered by most as a subgenus of Bembidion (e.g., Bousquet and Larochelle 1993; Lindroth 1963), has recently been removed from Bembidion (Lorenz 2005). Our results strongly indicate that Bembidion (Hydrium) levigatum is a Bembidion, and, among the taxa sampled, the sister group of Bembidion (Metallina) dyschirinum, with which it shares a number of characteristics, including an angulate shoulder margin.
Cillenus was considered by Lindroth (1980) and Toledano (2000) to be within Bembidion, but most current authors treat it as a separate genus (Lorenz 2005; Marggi et al. 2003; Ortuño and Toribio 2005; Perrault 1981). This separation from Bembidion is based in part on the unusual morphological traits of adult Cillenus, including a wide head and long mandibles. These features are likely derived features resulting from adaption to feeding on amphipods in their intertidal habitat (Green 1956; Lindroth 1980). Our results (Figs 2, 4, 5; Table 5) strongly support Cillenus as a member of Bembidion, near the Ocydromus complex.
When described, Lindroth (1980) separated Zecillenus, a lineage from New Zealand, from Bembidion because of the lack of a brush in the male aedeagus of Zecillenus. These beetles are rather distinct in general form, and have a number of unique characteristics, including unusual flanges on the elytra (Lindroth 1980). Our results indicate that they are highly derived Bembidion: among the taxa we sampled, they are strongly supported as belonging to Bembidon by all three genes and the merged matrix (Table 5), and are sister group to Bembidion (Zeplataphus) tairuense in 28S, wingless, and merged matrices. Bembidion tairuense is the only other Bembidion we have sampled from New Zealand, and we propose that Zecillenus is part of an endemic radiation of New Zealand Bembidion.
Relationships of the major lineages of trechitesThe only well-supported result we obtained about the relationships between the tribes or subtribes of trechites was the sister-group relationship between Tachyina and Xystosomina. This relationship is supported by the common presence of a recurrent groove on the elytra (Erwin 1994: 558).
ConclusionsWhile our results have clarified the position of a number of enigmatic lineages within Trechitae, including Tasmanitachoides, Cillenus, and Zecillenus, our data have surprisingly little to say about deep relationships within Trechitae (Fig. 7). This is perhaps a result of the shortness of the deep branches in ribosomal gene trees (Figs 2, 3). Whether these might indicate a rapid radiation or limited ribosomal evolution during that period, they make inference of the relationships difficult.
Future work should increase taxon sampling, to split long branches in the tree, and add missing lineages. Additional genes, especially those with relatively longer lengths for the deeper branches (such as those seen in wingless, Fig. 4), are also needed. These efforts should increase our understanding of the phylogeny of this diverse group of small beetles.
Our heartfelt thanks go to Ross and Joyce Bell, who have provided inspiration over the years to both of us, through their excellent work and generous natures.
Many people aided this project by providing valuable specimens, and we thank them all: David Kavanaugh, Alfred Newton, Margaret Thayer, Wendy Moore, Geoff Monteith, Wayne Maddison, Konjev Desender, Betsy Arnold, Julia Amerongen Maddison, A.S. Gerber, P.M. Johns, Allan Ashworth, Matthias Hartmann, J. M. Pérez Zaballos, J.I. Townsend, Wolfgang Dormann, Dietrich Mossakowski, D.J. Cook, S. Endrödy-Younga, O. Junco, W. Reeves, Rich Leschen, Chuck Bellamy, Karl Kjer, Roger Blahnik, Vasily Grebennikov, and Kipling Will.
For help in identifying specimens, we thank Terry Erwin, Konjev Desender, J. M. Pérez Zaballos, Matthias Hartmann, Paolo Bonavita, Geoff Monteith, Igor Sokolov, and Vasily Grebennikov.
I thank Vasily Grebennikov for making the original data matrix available from his 2008 study. Thanks as well to the Willi Hennig Society for making TNT available through its sponsorship.
This project was funded primarily by NSF grants DEB-9420219 and DEB-9981935 to DRM, as well as funds generously provided by the University of Arizona, and the Harold E. and Leona M. Rice Endowment Fund at Oregon State University.
Locality data for specimens sequenced in this study. # is the D.R. Maddison voucher number.
| Taxon | # | Locality |
|---|---|---|
| Moriomorphini | ||
| Sitaphe parallelipennis | 0669 | Australia: North Queensland: Devil’s Thumb via Mossman |
| Sitaphe parallelipennis | 2247 | Australia: North Queensland: Upper Whitehall Gully |
| Tropopterus sp. | 2200 | Chile: Reg. IX: Parque Nacional Nahuelbuta |
| Psydrini | ||
| Psydrus piceus | 1627 | USA: New Mexico: Gila National Forest, Pine Flats Campground |
| Nomius pygmaeus | 0893 | USA: Arizona: Pima Co., Rincon Peak |
| Trechini: Trechodina | ||
| Cnides sp. | 1808, 0691 | México: Sonora: Alamos, Rio Cuchujaqui |
| Pachydesus sp. | 0678 | Republic of South Africa: Kwazulu-Natal, Ngome Forest Reserve |
| Perileptus areolatus | 1707 | Spain: Madrid: Rio Cofio |
| Perileptus areolatus | 0824 | Russia: NW Caucasus: Krasnodar Dist., r. Belaya, Nikel |
| Perileptus sloanei | 0767 | Australia: Queensland: Gray’s Waterhole, Gayndah. |
| Thalassophilus longicornis | 0823 | Russia: NW Caucasus: Krasnodar Dist., r. Belaya, Nikel |
| Trechodes bipartitus | 0705 | Australia: Queensland: Cow Bay |
| Trechodes jeanneli jeanneli | 0606 | Madagascar: Fianarantsoa Province: Ranomafana National Park |
| Trechosiella sp. | 1709 | Republic of South Africa: North Cape Prov.: Farm Klipdam |
| Tasmanitachoides fitzroyi | 1575, 0762 | Australia: Queensland: Gayndah, Gray’s Waterhole |
| Trechini: Trechina | ||
| Trechus oregonensis | 0587 | USA: Montana: Glacier Co., Divide Creek |
| Omalodera limbata | 0571 | Chile: Malleco Pr.: Coimallin area, 8.2 km NW Los Portones |
| Homaloderodes germaini | 1066 | Chile: Malleco Pr.: Coimallin area, 8.2 km NW Los Portones |
| Kenodactylus audouini | 0670 | Argentina: Tierra Del Fuego: 78 km E. of Ushuaia. |
| Paratrechus sp. | 1076 | Costa Rica: Cerro de la Muerte |
| Trechinotus flavocinctus | 0575 | Chile: Palena Pr.: Austral Highway km 67.9 (11 km S. Contao turnoff) |
| Zolini | ||
| Merizodus angusticollis | 0453 | Chile: Valdivia Province: Rincón de la Piedra, 14.8 km SE Valdivia |
| Oopterus sp. | 0387 | New Zealand: South Island, Canterbury Prov, Arthur’s Pass Nat. Park |
| Oopterus helmsi | 0354 | New Zealand: South Island, Canterbury Prov, Arthur’s Pass Nat. Park |
| Sloaneana tasmaniae | 0339 | Australia: Tasmania: Mount Field N.P., Lake Dobson Rd., E end Wombat Moor |
| Pogonini | ||
| Pogonus (Pogonus) chalceus | 1711, 0679 | Spain: Cádiz: El Puerto de Sta. Maria |
| Thalassotrechus barbarae | 0703, 0530 | USA: California: Marin Co., Tiburon Peninsula |
| Bembidiini: Tachyina | ||
| Lymnastis sp. | 0988 | Malaysia: Sabah: Kinabatangan River |
| Micratopus sp. | 0605 | USA: Arizona: Pima Co., Tucson |
| Paratachys vorax | 0410 | USA: Arizona: Santa Cruz Co., Santa Cruz River near Tumacacori |
| Elaphropus obesulus | 0411 | USA: Arizona: Santa Cruz Co., Santa Cruz River near Tumacacori |
| Elaphropus cf. nigrolimbatus | 0761 | Republic of South Africa: Kwa-Zulu-Natal: Near Bayala |
| Elaphropus sp. 3 | 0713 | Republic of South Africa: North Cape Prov.: Farm Klipdam |
| Pericompsus laetulus | 0429 | USA: Arizona: Pima Co., Arivaca Creek |
| Polyderis rufotestacea | 0717, 0718 | USA: Arizona: Gila Co., Gila River near Winkelman |
| Tachys vittiger | 0760 | USA: California: San Diego Co., Batiquitos Lagoon |
| Tachys corax | 0604 | USA: Arizona: Gila Co., Winkelman |
| Tachyta nana inornata | 0573 | USA: Mississippi: Noxubee Co., Noxubee Nat. Wildlife Refuge |
| Bembidiini: Xystosomina | ||
| Erwiniana hilaris | 0409 | Ecuador: Sucumbios: Cuyabeno Faunal Reserve |
| Erwiniana crassa | 0989 | Ecuador: Orellana Province: Tiputini |
| Mioptachys flavicauda | 0684 | USA: Mississippi: Noxubee Co., Noxubee Nat. Wildlife Refuge |
| Philipis bicolor | 0592 | Australia: Queensland: Mt. Lewis Rd |
| Bembidiini: Anillina | ||
| Anillinus (langdoni group) sp. | 0690 | USA: Georgia: Habersham Co., Big Panther Creek Trail |
| Serranillus sp. | 1084 | USA: North Carolina: Graham Co., Santeetlah Lake |
| Typhlocharis armata | 0572, 1718 | Spain: Cádiz: Tarifa |
| Nesamblyops sp. | 0696 | New Zealand: 3.5 km N Rapahoe |
| Bembidiini: Bembidiina | ||
| Asaphidion alaskanum | 0585 | USA: Alaska: mile 412.3 Dalton Highway |
| Asaphidion championi | 0574 | Nepal: Prov. Karnali, Dolpo, Tripurakot Flußufer |
| Asaphidion curtum | 0267 | USA: Massachusetts: Norfolk Co., Jamaica Plain |
| Amerizus sp. | 0576 | USA: Utah: Abajo Mountains |
| Ocys harpaloides | 0569 | Belgium: Schorisse |
| Phrypeus rickseckeri | 0776 | USA: California: Del Norte Co., Smith River |
| Phrypeus rickseckeri | 0692 | USA: Montana: Jefferson Co., Boulder River at Galena Gulch |
| Sinechostichus solarii | 0603 | Italy: Tuscany: Vallombrosa |
| Bembidion (Antiperyphanes)sp. nr. chilense | 0714 | Peru: Pisac, tributary of Rio Urubamba, 3020m |
| Bembidion (Hoquedela) cf. csikii | 0916 | China: Yunnan Prov.: Gaoligong Shan, Nujiang Prefecture, 13.5 air km SSW of Gof Gonshan |
| Bembidion (Cillenus) laterale | 0602 | Germany: Wadden Sea, Mellum Island |
| Bembidion (Notaphus) insulatum | 0444 | USA: Arizona: Cochise Co., 2.2 km S of Willcox |
| Bembidion (Metallina) dyschirinum | 0896 | USA: Washington: Columbia Co., Blue Mountains |
| Bembidion (Hydrium) levigatum | 0763 | USA: Nebraska: Lancaster Co., Lincoln |
| Bembidion (Ocydromus) mexicanum | 0260 | USA: Arizona: Cochise Co., Turkey Creek, Chiricahua Mtns |
| Bembidion (Phyla) obtusum | 0895 | Canada: Ontario: Burlington |
| Bembidion (Melomalus) planatum | 0601 | Canada: British Columbia: Alexander Creek on highway 3 |
| Bembidion (Bembidion) quadrimaculatum dubitans | 0676 | Canada: Alberta: Bayette Lake near Flatbush |
| Bembidion (Zeplataphus) tairuense | 0607 | New Zealand: Tekapo River Delta, Lake Benmore |
| Bembidion (Zecillenus)sp. | 0595 | New Zealand: Foxton Beach, Manuwatu |
| Taxon | # | Locality |
|---|---|---|
| Moriomorphini | ||
| Sitaphe parallelipennis | 0669 | Australia: North Queensland: Devil’s Thumb via Mossman |
| Sitaphe parallelipennis | 2247 | Australia: North Queensland: Upper Whitehall Gully |
| Tropopterus sp. | 2200 | Chile: Reg. IX: Parque Nacional Nahuelbuta |
| Psydrini | ||
| Psydrus piceus | 1627 | USA: New Mexico: Gila National Forest, Pine Flats Campground |
| Nomius pygmaeus | 0893 | USA: Arizona: Pima Co., Rincon Peak |
| Trechini: Trechodina | ||
| Cnides sp. | 1808, 0691 | México: Sonora: Alamos, Rio Cuchujaqui |
| Pachydesus sp. | 0678 | Republic of South Africa: Kwazulu-Natal, Ngome Forest Reserve |
| Perileptus areolatus | 1707 | Spain: Madrid: Rio Cofio |
| Perileptus areolatus | 0824 | Russia: NW Caucasus: Krasnodar Dist., r. Belaya, Nikel |
| Perileptus sloanei | 0767 | Australia: Queensland: Gray’s Waterhole, Gayndah. |
| Thalassophilus longicornis | 0823 | Russia: NW Caucasus: Krasnodar Dist., r. Belaya, Nikel |
| Trechodes bipartitus | 0705 | Australia: Queensland: Cow Bay |
| Trechodes jeanneli jeanneli | 0606 | Madagascar: Fianarantsoa Province: Ranomafana National Park |
| Trechosiella sp. | 1709 | Republic of South Africa: North Cape Prov.: Farm Klipdam |
| Tasmanitachoides fitzroyi | 1575, 0762 | Australia: Queensland: Gayndah, Gray’s Waterhole |
| Trechini: Trechina | ||
| Trechus oregonensis | 0587 | USA: Montana: Glacier Co., Divide Creek |
| Omalodera limbata | 0571 | Chile: Malleco Pr.: Coimallin area, 8.2 km NW Los Portones |
| Homaloderodes germaini | 1066 | Chile: Malleco Pr.: Coimallin area, 8.2 km NW Los Portones |
| Kenodactylus audouini | 0670 | Argentina: Tierra Del Fuego: 78 km E. of Ushuaia. |
| Paratrechus sp. | 1076 | Costa Rica: Cerro de la Muerte |
| Trechinotus flavocinctus | 0575 | Chile: Palena Pr.: Austral Highway km 67.9 (11 km S. Contao turnoff) |
| Zolini | ||
| Merizodus angusticollis | 0453 | Chile: Valdivia Province: Rincón de la Piedra, 14.8 km SE Valdivia |
| Oopterus sp. | 0387 | New Zealand: South Island, Canterbury Prov, Arthur’s Pass Nat. Park |
| Oopterus helmsi | 0354 | New Zealand: South Island, Canterbury Prov, Arthur’s Pass Nat. Park |
| Sloaneana tasmaniae | 0339 | Australia: Tasmania: Mount Field N.P., Lake Dobson Rd., E end Wombat Moor |
| Pogonini | ||
| Pogonus (Pogonus) chalceus | 1711, 0679 | Spain: Cádiz: El Puerto de Sta. Maria |
| Thalassotrechus barbarae | 0703, 0530 | USA: California: Marin Co., Tiburon Peninsula |
| Bembidiini: Tachyina | ||
| Lymnastis sp. | 0988 | Malaysia: Sabah: Kinabatangan River |
| Micratopus sp. | 0605 | USA: Arizona: Pima Co., Tucson |
| Paratachys vorax | 0410 | USA: Arizona: Santa Cruz Co., Santa Cruz River near Tumacacori |
| Elaphropus obesulus | 0411 | USA: Arizona: Santa Cruz Co., Santa Cruz River near Tumacacori |
| Elaphropus cf. nigrolimbatus | 0761 | Republic of South Africa: Kwa-Zulu-Natal: Near Bayala |
| Elaphropus sp. 3 | 0713 | Republic of South Africa: North Cape Prov.: Farm Klipdam |
| Pericompsus laetulus | 0429 | USA: Arizona: Pima Co., Arivaca Creek |
| Polyderis rufotestacea | 0717, 0718 | USA: Arizona: Gila Co., Gila River near Winkelman |
| Tachys vittiger | 0760 | USA: California: San Diego Co., Batiquitos Lagoon |
| Tachys corax | 0604 | USA: Arizona: Gila Co., Winkelman |
| Tachyta nana inornata | 0573 | USA: Mississippi: Noxubee Co., Noxubee Nat. Wildlife Refuge |
| Bembidiini: Xystosomina | ||
| Erwiniana hilaris | 0409 | Ecuador: Sucumbios: Cuyabeno Faunal Reserve |
| Erwiniana crassa | 0989 | Ecuador: Orellana Province: Tiputini |
| Mioptachys flavicauda | 0684 | USA: Mississippi: Noxubee Co., Noxubee Nat. Wildlife Refuge |
| Philipis bicolor | 0592 | Australia: Queensland: Mt. Lewis Rd |
| Bembidiini: Anillina | ||
| Anillinus (langdoni group) sp. | 0690 | USA: Georgia: Habersham Co., Big Panther Creek Trail |
| Serranillus sp. | 1084 | USA: North Carolina: Graham Co., Santeetlah Lake |
| Typhlocharis armata | 0572, 1718 | Spain: Cádiz: Tarifa |
| Nesamblyops sp. | 0696 | New Zealand: 3.5 km N Rapahoe |
| Bembidiini: Bembidiina | ||
| Asaphidion alaskanum | 0585 | USA: Alaska: mile 412.3 Dalton Highway |
| Asaphidion championi | 0574 | Nepal: Prov. Karnali, Dolpo, Tripurakot Flußufer |
| Asaphidion curtum | 0267 | USA: Massachusetts: Norfolk Co., Jamaica Plain |
| Amerizus sp. | 0576 | USA: Utah: Abajo Mountains |
| Ocys harpaloides | 0569 | Belgium: Schorisse |
| Phrypeus rickseckeri | 0776 | USA: California: Del Norte Co., Smith River |
| Phrypeus rickseckeri | 0692 | USA: Montana: Jefferson Co., Boulder River at Galena Gulch |
| Sinechostichus solarii | 0603 | Italy: Tuscany: Vallombrosa |
| Bembidion (Antiperyphanes)sp. nr. chilense | 0714 | Peru: Pisac, tributary of Rio Urubamba, 3020m |
| Bembidion (Hoquedela) cf. csikii | 0916 | China: Yunnan Prov.: Gaoligong Shan, Nujiang Prefecture, 13.5 air km SSW of Gof Gonshan |
| Bembidion (Cillenus) laterale | 0602 | Germany: Wadden Sea, Mellum Island |
| Bembidion (Notaphus) insulatum | 0444 | USA: Arizona: Cochise Co., 2.2 km S of Willcox |
| Bembidion (Metallina) dyschirinum | 0896 | USA: Washington: Columbia Co., Blue Mountains |
| Bembidion (Hydrium) levigatum | 0763 | USA: Nebraska: Lancaster Co., Lincoln |
| Bembidion (Ocydromus) mexicanum | 0260 | USA: Arizona: Cochise Co., Turkey Creek, Chiricahua Mtns |
| Bembidion (Phyla) obtusum | 0895 | Canada: Ontario: Burlington |
| Bembidion (Melomalus) planatum | 0601 | Canada: British Columbia: Alexander Creek on highway 3 |
| Bembidion (Bembidion) quadrimaculatum dubitans | 0676 | Canada: Alberta: Bayette Lake near Flatbush |
| Bembidion (Zeplataphus) tairuense | 0607 | New Zealand: Tekapo River Delta, Lake Benmore |
| Bembidion (Zecillenus)sp. | 0595 | New Zealand: Foxton Beach, Manuwatu |