(C) 2012 David M. General. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
An overview of the history of myrmecology in the Philippine archipelago is presented. Keys are provided to the 11 ant subfamilies and the 92 ant genera known from the Philippines. Eleven ant genera (12%), including 3 undescribed genera, are recorded for the first time from the Philippines. The biology and ecology of the 92 genera, illustrated by full-face and profile photo-images, of Philippine ants are summarized in the form of brief generic accounts. A bibliography of significant taxonomic and behavioral papers on Philippine ants and a checklist of valid species and subspecies and their island distributions are provided.
Formicidae, Philippines, ant genera, keys, new records, species list
The study of ants can be difficult, particularly in the tropics. Tropical ant faunas are only partly explored and information concerning them is widely scattered throughout the scientific literature. Taxonomic references are frequently hard to access, badly outdated, and difficult for the non-specialist to use. The systematics of several major genera (e.g., Camponotus, Crematogaster, and Pachycondyla) is still chaotic and unrecognized synonyms and undescribed species abound. Taxonomic progress is relatively slow because most type material of described material is deposited in Europe, North America, or Japan, and museums are understandably hesitant to send fragile types to workers in the developing world (Naskrecki 2004).
Introductory accounts of important tropical ant faunas are badly needed to provide an entry point for biologists interested in studying ants. The purpose of this paper is to provide such an introduction to the ant fauna of the Philippines.
Simplified keys to subfamilies and to genera are presented. Most character states in the keys are discernible with a 40X stereomicroscope. Technical terms and subtle character states are minimized. Full-face and profile color photo-images are included to illustrate the different genera found in the Philippines. A glossary is also included to help the student understand the precise technical terms used in the keys. And the most up-to-date systematic arrangement of genera is used (Bolton et al. 2006, Bolton 2011). We hope that this paper will stimulate local interest in the study of Philippine ants.
A short history of Philippine myrmecologyThe study of Philippine ant fauna began in the second half of the 1800’s, during the last century of the Spanish colonial period. Small numbers of Philippine ants were collected by European travelers who then sold or gave their collections to the great myrmecologists of that era, notably Auguste Forel and Carlo Emery. Emery described ants collected in Manila and Antipolo, Luzon Island by E. Simon, a Frenchman (Emery 1893c). Frederick Smith described a few species from the Philippines without specifying the provenance of the specimens (F. Smith 1858). These researchers described some new species, but made no effort to characterize the Philippine ant fauna as a whole.
During the early American colonial era, Richard C. MacGregor, a US-trained Australian ornithologist, Charles S. Banks and other biologists working for the Bureau of Science, Manila (Van Steenis-Kruseman 2006), traveled the archipelago and gave or sold their insect collections to Americans like Dr. W.H. Ashmead of the United States National Museum (USNM) and the great Dr. William Morton Wheeler of Harvard University. Ashmead described new species collected by Dr. P.L. Stangl of the U.S. Army, Prof. L.E. Griffin of Missouri Valley University, Dr. E.B. Copeland of the Government Laboratories in Manila, and Dr. M.H. Smith of the US Fish Commission (Ashmead 1904a, 1904b). Dr. Francis X. Williams, who studied under W.M. Wheeler, collected ants while he was looking for insect predators of agricultural pests, as a researcher for the USDA. He took advantage of the expertise of Dr. Charles F. Baker, a professor at the University of the Philippines College of Agriculture at Los Baños, Laguna. Baker collected insects prodigiously, amassing hundreds of Schmitt boxes, which were bequeathed to USNM (Evans 1985).
The first intensive ant collecting was done by Dr. James W. Chapman, Wheeler’s colleague at Harvard, who arrived in the 1910’s to be a missionary and teacher at Silliman University in Dumaguete, Negros Oriental Province, Negros Island. Chapman concentrated on studying the ants of the Cuernos de Negros Mountains, but also collected in northern Luzon and Mindanao. During the Second World War, he continued to collect ants even as Japanese soldiers were hunting him down. Fortunately, he survived capture and incarceration, and his collection, which he had hidden, was largely intact (Chapman and Chapman 1947). Most of these specimens, still in their original jars, are deposited in the Museum of Comparative Zoology (MCZ) at Harvard University. Chapman later published a list of the ants of Asia (Chapman and Capco 1951).
Interestingly, Jesuit priests were important early collectors of Hymenoptera, including ants. Ashmead studied the specimens collected by Fr. W.A. Stanton, S.J. and Fr. Robert E. Brown, S.J. (Ashmead 1904a, 1904b). Fr. Brown collected the holotype queen of the controversial genus Pseudaphomomyrmex, recently synonymized under the genus Tapinoma (Ashmead 1905, LaPolla and Longino 2006, Fisher and Bolton 2007). Fr. B.B. Lowery S.J. also collected ants in the Philippines in the 1960s (Rigato 1994, Ward 2001, Shattuck 2011). His collections have been studied by researchers all over the world.
During the Commonwealth period and after the Second World War, some Filipinos also collected ants. This fact can be gleaned from the locality labels on specimens in certain museums. Specimens collected by Domingo Empeso, H.M. Torrevillas, A. Reyes, and M. Ramos are in the collections of the MCZ and the Bernice P. Bishop Museum in Hawaii. In 1966, Dr. Clare Baltazar published a monograph on Philippine Hymenoptera, which included 235 entries for ant species (Baltazar 1966).
Current status of Philippine myrmecologySince the efforts of the late Dr. James W. Chapman in the 1920s to 1940s, there has been relatively little intensive collecting and the studies of ants in ecological research have been few (Calilung 2000, Caceres-Plopenio, unpubl. M.S. thesis). A recent transect study (Alpert and General, in prep.) surveyed the ants of a 27-year-old narra private reforestation project on the slopes of Mt. Isarog in the Bicol region of Luzon Island. New generic records and new species are recorded. A subsequent transect study by Joanaviva Caceres-Plopenio in a nearby area added more species distribution records and possibly new species (Caceres-Plopenio, unpubl. M.S. thesis; K. Eguchi, pers. comm.). More recently, Perry Buenavente conducted ant surveys on several mountains in Luzon and Mindanao during expeditions of the National Museum of the Philippines, accumulating many new collections (P. Buenavente, pers. comm.).
Inventories for undergraduate and graduate theses remain an important source of specimens, especially in unexplored study sites. Fortunately, the collection methods are now more standardized (Agosti et al. 2000), allowing the comparison of datasets from different localities. Government permits are required for any collecting of specimens on Philippine soil and export of the same. And the Philippine government is the perpetual owner of all specimens, including holotype specimens.
The study of Philippine ant diversity is still in its infancy. A cursory examination of the species list (see Appendix) shows a strong sampling bias in the known distribution of species. Luzon Island has at least 265 species while Mindanao Island, only slightly smaller in area, has but 99 recorded species. Neither island is close to being well-collected. The large islands in the central Philippines, with the exception of Negros, namely Leyte, Mindoro, Panay, and Samar, are very poorly represented in the list. Any attempt at understanding the biogeography of Philippine ants is therefore premature.
Perhaps the best documented ant fauna in the Philippines is that of the Cuernos de Negros Mountains near Dumaguete City, Negros Island. This area was the favorite hunting ground of Chapman, who collected there for about two decades (Chapman and Chapman 1947). Nevertheless, with only 123 species known from Negros Island, more intensive and systematic collection methods will certainly turn up new species and new distributional records there.
Much has changed since the publication of Baltazar’s monograph. Many of the genera have been revised, many species have been added to the list, and a number of names have been synonymized. This paper presents an updated list containing 474 valid species and subspecies names for ants in the Philippines.
Recent taxonomic contributions relevant to the study of Philippine antsThere has been some recent taxonomic progress. Recent monographs relevant to the Philippine ant fauna include: Acanthomyrmex (Moffett 1986, Agosti 1992), Acropyga (LaPolla 2004), Anillomyrma (Eguchi et al. 2010), Anochetus (Zettel 2012) Calyptomyrmex (Shattuck 2011), Cardiocondyla (Seifert 2003), Carebara (Fernandez 2004), Euprenolepis (LaPolla 2009), Forelophilus (Zettel and Zimmerman 2007), Gnamptogenys of the Old World (Lattke 2004), Iridomyrmex (Heterick and Shattuck 2011), Leptomyrmex (Lucky and Ward 2010), Liomyrmex (Rigato and Bolton 2001), Lordomyrma (Taylor 2012), Lophomyrmex (Rigato 1994), Mayriella (Shattuck and Barnett 2007), Meranoplus (Schödl 1998), Monomorium (Heterick 2001), Myrmoteras (Moffett 1985, Agosti 1992, Zettel and Sorger 2011), Mystrium (Bihn and Verhaagh 2007), Odontomachus (Sorger and Zettel 2011), Paratopula (Bolton 1988), Prionopelta (Shattuck 2008a), Pristomyrmex (Wang 2003, Zettel 2006), Proceratium (Baroni Urbani and de Andrade 2003), Probolomyrmex (Eguchi et al. 2006), Pyramica (now also Strumigenys sensu Baroni Urbani and de Andrade 2007)(Bolton 2000), Recurvidris (Bolton 1992, Zettel 2008), Rhoptromyrmex (Bolton 1986), Strumigenys (Bolton 2000), Technomyrmex (Bolton 2007), Tetraponera (Ward 2001) and Vombisidris (Zettel and Sorger 2010a).
Why study ants in the Philippines?The high diversity of ants in the Philippines makes inventory studies interesting, with little of the monotony of encountering the same species over and over again. Ant surveys can detect the presence of invasive species and provide insights into the biogeography of the islands, a baseline for pre-operation inventories of mining sites and a measure of post-mining or post-logging remediation. There is much to be done and discovered, and many opportunities await the Filipino myrmecologist.
There are many interesting study sites in the Philippines. Particularly interesting are: old growth forests, old mangrove areas, small islands (preferably uninhabited), wooded ravines, reforested areas, and even microhabitats such as soil and forest canopies. Each island or province has its own opportunities. The ant faunas of the large central islands remain unexplored. Surprisingly, new species have been found in even the most disturbed localities, such as university campuses (DMG, unpub. notes), possibly relicts of a more diverse ant assemblage (S. Cover, pers. comm.).
Geographical and bio-climatic features of the PhilippinesThe Philippines is so ecologically diverse that it is very difficult to characterize the country in a single paragraph. While the climate is generally tropical and maritime, there are zones which are distinctly different. The mean temperature ranges from 25.5° C in January to 28.3° C in May, however, high-elevation locations (>1, 000 masl) are sub-temperate. Baguio City, in northern Luzon, has a mean annual temperature of only 18.3˚C. Some parts, such as southern Cotabato Province, Min- danao Island, are relatively dry with an average annual rainfall of only 978 mm (PAGASA 2010). In contrast, 5713 mm of rainfall was measured at 1650 masl on Mt. Isarog in 114 days from November 1993 to May 1994 (Heaney et al. 1999). There are also wet tropical zones, mainly in the eastern part of the country, and dry tropical zones that are in the rain shadow (west) of mountain ranges. Camarines Sur, a province in the Bicol Peninsula of Luzon Island, for instance, has three distinct climate patterns, depending on elevation and position relative to Mt. Isarog, the dominant feature of the landscape. There is also a typhoon corridor, mainly in the eastern part of the archipelago, which is visited by an average of 19 typhoons every year (PAGASA 2010).
The Philippines is composed of over 7, 100 islands, most of which are uninhabited. There are islands, such as Sibuyan Island in the central Philippines, which have never been connected to larger islands, even during the last Ice Age (Heaney et al. 1999). Single mountains, usually volcanoes, also exist as island habitats within the broader lowlands. This complex blend of climatic diversity and opportunity for geographic isolation has likely led to high levels of endemism, a phenomenon that deserves much further exploration. For example, two adjacent islands, Biliran and Samar, each have their own species of Meranoplus, a ground-dwelling myrmicine ant (Schödl 1998; DMG, unpub. notes). The Palawan island group is unique in that many of its plants and animals, including some ants, are found nowhere else in the Philippines, or in the world. Palawan Island is the type locality of the enigmatic leptanilline genus Noonilla.
Threats to Philippine ant diversityThe Philippines is considered one of the hottest of the biodiversity hotspots because of the severe human pressure on its highly endemic native flora and fauna. Time is running out for the the remaining primary forests. Despite decades of regulation and reforestation, forests continue to dwindle. For instance, all the privately reforested narra trees (Fabaceae: Pterocarpus indicus Willd.) of the study site of our 2003-4 transects (Alpert and General in prep.) had already been poached by 2009, wiping out 27 years of stewardship of the area. Even the stumps were removed (DMG, pers. obs.). Forest destruction seems to go on unabated, exacerbated by new large-scale mining projects that seem to target the mineral resources under primary forests.
Aside from habitat destruction, invasive ant species also impact the native ant assemblages, although the extent and severity of this influence is not known. In both urban and agricultural areas are found the worldwide invasive species, e.g., Solenopsis geminata (Fabricius 1804), Tapinoma melanocephalum (Fabricius 1793), and Paratrechina longicornis (Latreille 1802). Other invasive species have not been found, but this may be due to a lack of attention.
Some forest habitats in the PhilippinesWe include some images of intact and heavily damaged forests to illustrate the potentials and problems for ant research in the Philippines. The Philippine ant fauna is very poorly explored and records are few and spotty at best. A researcher can essentially choose a mountain among several hundred mountains, get the necessary permits and be the first one to study the ants on that mountain. However, that mountain may also be very disturbed or degraded, leaving only the tramp species that abound in disturbed habitats. In addition to environmental damage, there is also an active communist insurgency and other serious security threats to researchers in the field. Other areas may harbor malaria mosquitoes and other serious health threats. Hence, local collaboration is quite necessary to minimize the risks of field work in the Philippines.
Genus accounts of Philippine ant generaIn this contribution, the acronym “PH” is used to represent the archipelago of the Republic of the Philippines, in compliance with the International Organization for Standardization 3166-1 country codes (ISO 2010). The archipelago should also be referred to as “the Philippines” not “the Philippine Islands”. The expression “Philippine Islands” and the acronym “PI” are anachronisms and should no longer be used since they refer to the islands before the country gained independence in 1946.
The first electronic checklist of Philippine ants is available online at Discoverlife (Alpert et al. 2006). Another online resource is the Philippine page on AntWeb, hosted by the California Academy of Sciences (Alpert and General 2008).
Montane forest, Mt. Palali, Sierra Madre Mountain Range, Luzon Island. Image used with permission from Arvin C. Diesmos.
Montane forest, Mt. Palali, Sierra Madre Mountain Range, Luzon Island. Image used with permission from Arvin C. Diesmos.
Slash and burn agriculture, Sierra Madre Mountain Range, Luzon Island. Image used with permission from Arvin C. Diesmos.
Slash and burn agriculture, Sierra Madre Mountain Range, Luzon Island. Image used with permission from Arvin C. Diesmos.
Beach forest, Aurora Province, Luzon Island. Image used with permission from Arvin C. Diesmos.
Beach forest, Aurora Province, Luzon Island. Image used with permission from Arvin C. Diesmos.
Deforested mountain, northwestern Mindoro Island.
Mossy forest, Mt. Balatukan, northeastern Mindanao Island. Image used with permission from Perry Buenavente.
Mossy forest, Mt. Balatukan, northeastern Mindanao Island. Image used with permission from Perry Buenavente.
Lowland dipterocarp forest, Bucas Grande Island, Surigao del Norte Province.
Lowland dipterocarp forest, at the mouth of the Puerto Princesa Subterranean River, St. Paul Bay, Puerto Princesa City, Palawan.
Lowland dipterocarp forest, at the mouth of the Puerto Princesa Subterranean River, St. Paul Bay, Puerto Princesa City, Palawan.
Limestone karst forest, St. Paul Bay, Puerto Princesa City, Palawan.
Because these are genus accounts, plural terms, e.g., “these ants” or “they” refer to species belonging to the genus. Singular terms, e.g., “this species” or “it” refers to a particular species in the genus. The genus account typically introduces Philippine material referable to the particular genus, then gives a brief description of the morphology or behavior of the ants belonging to the genus and finally suggests collecting techniques. Some behavioral aspects may be inferred from published studies of non-Philippine congeners.
Genus Acanthomyrmex Emery, 1893aMyrmicinae: 17 spp, 1 known from PH.
Figs. 9 A, B
The single known valid Philippine species, Acanthomyrmex mindanao Moffett, 1986, was described from specimens collected under bark in Mt. McKinley, Davao Region, southern Mindanao Island. Other specimens were collected in northern Mindanao Island: Momungan, Olangon, and Iligan City, Lanao del Sur Province and Gingoog City, Misamis Oriental Province. This species is also known from Sarawak, Malaysia. These ants may be collected by sifting leaf litter, baiting with small seeds, and looking under bark. Keys to species: Moffett (1986) (World, revision), Agosti (1992) (World, revision), Terayama et al. 1998.
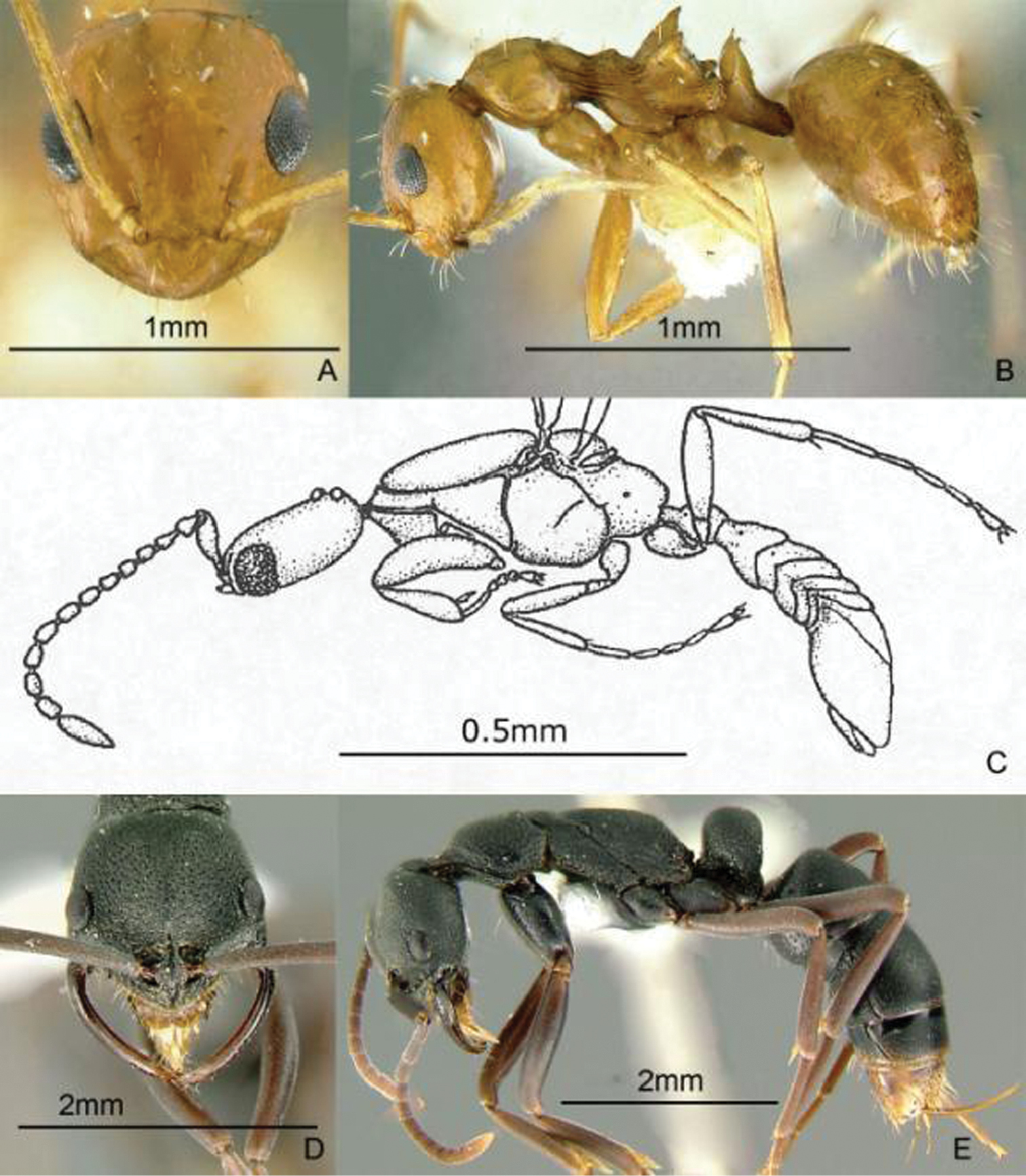
Full-face and profile images of Philippine ant genera. Acanthomyrmex mindanao, major worker A, B Acropyga pallida, minor worker C, D Aenictus ceylonicus (Mayr, 1866) E, F.
Full-face and profile images of Philippine ant genera. Acanthomyrmex mindanao, major worker A, B Acropyga pallida, minor worker C, D Aenictus ceylonicus (Mayr, 1866) E, F.
Formicinae: 40 spp., 3 known from PH.
Figs. 9 C, D
(New record). This genus is pantropical. These tiny, yellow, hypogaeic ants are known to tend subterranean coccids. One species known from the Philippines, Acropyga pallida (Donisthorpe, 1938), was originally described from New Guinea. These ants may be collected by sifting leaf litter and soil cores up to about 10 cm deep. Key to species: LaPolla (2004).
Genus Aenictus Shuckard, 1840Aenictinae: 149 spp., 15 known from PH.
Figs. 9 E, F
This genus is widespread throughout the archipelago. Large colonies forage for insect prey in the leaf litter. These are true army ants, making bivouacs in tree hollows and other protected cavities. These ants may be collected by searching for conspicuous columns of emigrating or foraging ants, and carefully inspecting cavities in trees and logs. For raiding behavior, see Schneirla and Reyes (1966). Keys to species: Wheeler (1930c) (Philippines), Wilson (1964) (Indo-Australian, revision), Terayama and Yamane (1989) (Indonesia, Sumatra), Shattuck (2008b) (Australian, review of genus), Zettel and Sorger (2010b) (new Philippine species described).
Genus Anillomyrma Emery, 1913bMyrmicinae: 2 spp., 1 known from PH.
Figs. 10 C, D
Specimens of Anillomyrma decamera (Emery, 1901) were recently collected by Joanaviva Caceres-Plopenio in a transect study on Mt. Isarog, Luzon Island (Caceres, unpubl. M.S. thesis; Eguchi et al. 2010). These pale, tiny, thin-skinned subterranean ants seem to prefer sandy soils. These ants may be collected by sifting soil cores, preferably by Berlese or Winkler extraction, or baiting underground in sandy loam areas. Key to species: Eguchi et al. 2010 (World, revision).
Full-face and profile images of Philippine ant genera. Stigmatomma luzonicum Wheeler and Chapman, 1925 (formerly Amblyopone luzonica)A, B Anillomyrma decamera (images reproduced with permission of Dr. Katsuyuki Eguchi) C, D Anochetus isolatus Mann, 1919 E, F.
Full-face and profile images of Philippine ant genera. Stigmatomma luzonicum Wheeler and Chapman, 1925 (formerly Amblyopone luzonica)A, B Anillomyrma decamera (images reproduced with permission of Dr. Katsuyuki Eguchi) C, D Anochetus isolatus Mann, 1919 E, F.
Ponerinae: 87 spp., 13 known from PH.
Figs. 10 E, F
These small, fast-moving trap-jawed ants are general predators and usually found foraging singly on the ground or in the leaf litter. These ants are similar to Odontomachus, except that they are smaller and have blunt petiolar nodes. Key to species: Brown (1978) (World), Zettel (2012) (Philippine species).
Genus Anomalomyrma Taylor, 1990aLeptanillinae: 2 spp., 1 known from PH.
Figs. 11 A, B
The worker caste of this enigmatic genus was recently discovered and described, including Anomalomyrma helenae Borowiec, Schulz, Alpert & Baňař, 2011 from northern Palawan Island (Borowiec et al. 2011). These tiny subterranean ants have a petiole and postpetiole whose tergites and sternites are fused to form rigid tubes, a unique morphology among ants. These ants may be collected by leaf litter sifting and underground baiting near water sources.
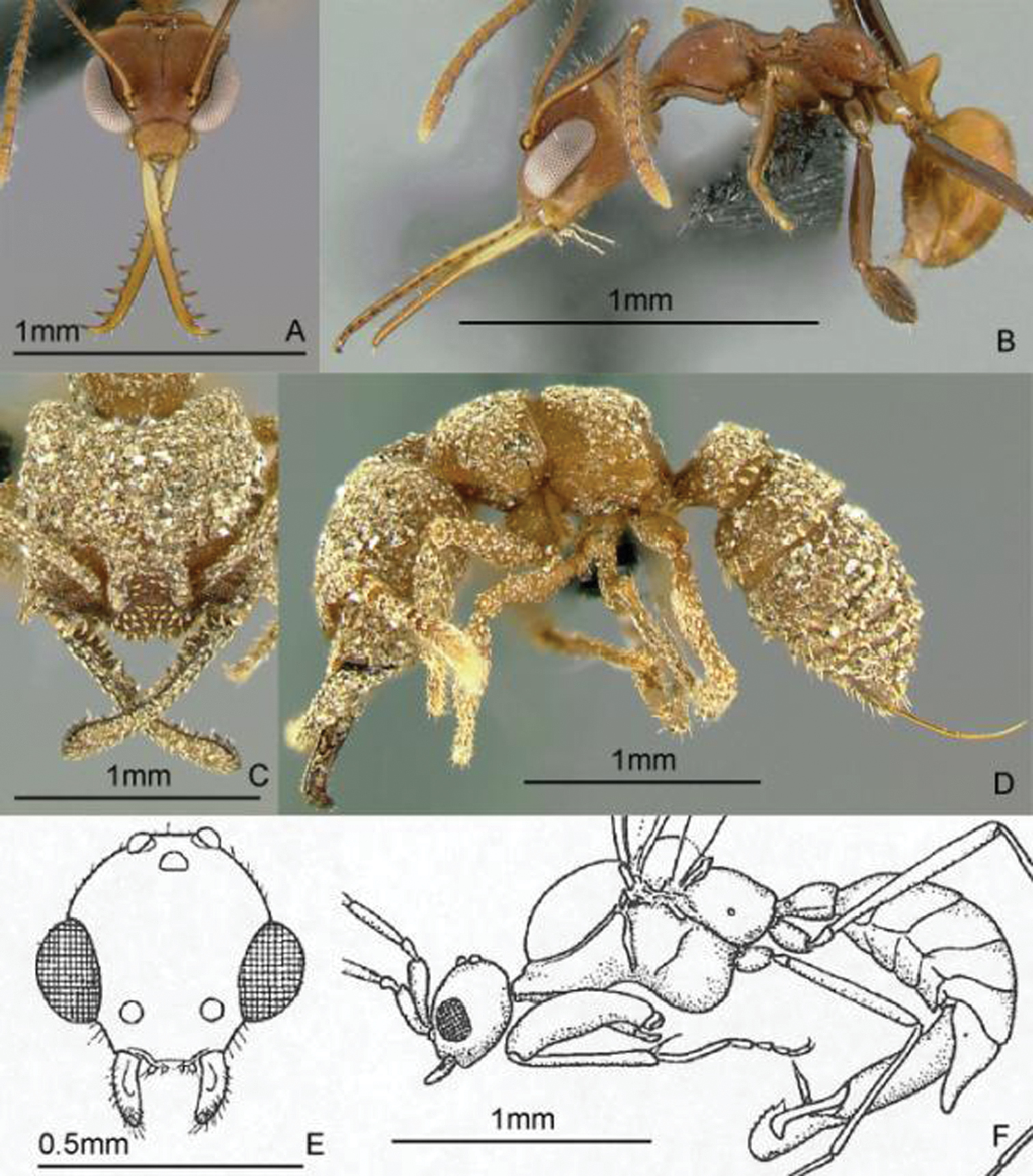
Full-face and profile images of Philippine ant genera. Anomalomyrma helenae A, B Anoplolepis gracilipes C, D Aphaenogaster species PH02 E, F.
Full-face and profile images of Philippine ant genera. Anomalomyrma helenae A, B Anoplolepis gracilipes C, D Aphaenogaster species PH02 E, F.
Formicinae: 14 spp., 1 known from PH.
Figs. 11 C, D
The species found in the Philippines, Anoplolepis gracilipes (F. Smith, 1857), is a pantropical invasive ant, with 11-segmented antennae with extremely long antennal scapes and a slender, constricted mesosoma in dorsal view. It is locally dominant around its nest. This species may nest in the ground or in a tree hollow. The myrmecophilous associations observed by the present authors indicate that this ant may be native to the Philippines. Upon opening a nest in a tree hollow, we found that muscoid flies immediately hovered over the scampering workers and the brood. In a coconut farm in Candelaria, Quezon Province, DMG also observed and collected an immature reduviid bug mimicking the erratic movement, color, and size of this ant. It may be collected by carefully inspecting tree hollows and rotten logs, baiting with tuna and searching for trails of foraging workers on the ground and along branches.
Genus Aphaenogaster Mayr, 1853Myrmicinae: 227 spp., 2 known from PH.
Figs. 11 E, F
(New record). There are historical specimens of two undescribed species, collected by Chapman and his field crew, of this genus in the ant collection of the Museum of Comparative Zoology, Harvard University. There are also new collections from Min-
danao Island (Figs. 11 E, F) (DMG, unpubl. notes). These ants typically nest in rotten logs and the soil under the logs. These ants may be collected by pitfall trapping, breaking into rotten logs and searching for foragers on the ground.
Genus Basiceros Schulz, 1906Myrmicinae: 60 spp., 3 known from PH.
Figs. 12 A, B
Ants of this genus may be confused with some Strumigenys species, but they have more than 6 antennal segments. These small, cryptic, slow-moving ants have clavate hairs all over the body. They forage in the leaf-litter. When disturbed, these ants roll up into a tight ball which makes them even harder to find. After about a minute, they stretch out and start walking slowly. There are specimens of an unidentified species from a transect study at Mt. Isarog, Bicol Region, Luzon Island (Alpert and General in prep.). Specimens were collected at 550 meters. These ants may be collected by sifting leaf litter over a white sheet, waiting a while, and carefully inspecting seedlike objects that begin to walk slowly. Keys to species (as Eurhopalothrix): Brown and Kempf (1960) (World); Taylor (1968, 1980, 1990b) (Indo-Australian, Australasian), Baroni Urbani and de Andrade 2007 (synonymy of Eurhopalothrix under Basiceros).
Full-face and profile images of Philippine ant genera. Basiceros philippinum (Brown and Kempf, 1960) A, B Calyptomyrmex beccarii Emery, 1887 C, D Camponotus albocinctus (Ashmead, 1905), major worker E, F.
Full-face and profile images of Philippine ant genera. Basiceros philippinum (Brown and Kempf, 1960) A, B Calyptomyrmex beccarii Emery, 1887 C, D Camponotus albocinctus (Ashmead, 1905), major worker E, F.
Myrmicinae: 25 spp., 2 known from PH.
Figs. 12 C, D
These small, cryptic, slow-moving ants bear a hard, thick integument with numerous evenly-spaced clavate hairs. They curl up into a ball when disturbed and are found in the leaf litter or on the ground. These ants may be collected by sifting leaf litter on a white sheet, waiting a while and carefully inspecting seed-like objects. After about a minute, these seed-like objects may stretch out and start to walk slowly. Keys: Baroni Urbani (1975) (Indian subcontinent); Bolton (1981) (Afrotropical); Shattuck (2011) (Southeast Asian revision).
Genus Camponotus Mayr, 1861Formicinae: 1, 584 spp., 30 known from PH.
Figs. 12 E, F
Camponotus is an extremely large genus in dire need of taxonomic revision. This is a widespread genus in the Philippines. Twenty-eight species are presently known from the Philippines, but this is probably only a fraction of the total. This genus is unusual among formicines in that the usual conspicuous ring of hairs around the acidipore is absent. This genus can be recognized by the placement of the antennal insertions, which are always set back (not adjacent to) from the posterior clypeal border. Camponotus are often medium to large ants; dimorphic or polymorphic workers that forage along trails from their nest in wood. Some species are nocturnal. Mimicry also occurs in this genus. There is a single undetermined minor worker in the Philippine collection of the Bernice P. Bishop Museum that looks very similar to ants of the genus Dolichoderus (GDA, unpub. notes). These ants may be collected by breaking into cavities in live wood or dead branches and searching for foragers on the ground, foliage, tree trunks and branches or in the canopy.
Genus Cardiocondyla Emery, 1869aMyrmicinae: 69 spp., 5 known from PH.
Figs. 13 A, B
This genus is known to have both normal winged and worker-like wingless males which fight and exhibit interesting courtship behavior (Heinze et al. 1993, Yamauchi et al. 2005, Mercier et al. 2007). These tiny, ground- and rock-dwelling ants bear the characteristic swollen postpetiole which is wider than long, and much larger than the petiole. Several species are pantropical tramps. There are 5 species known from the Philippines. One species, Cardiocondyla sima Wheeler, 1935b, is apparently widespread, reported in Lanao Province, Mindanao Island and in Mt. Isarog, Bicol Region, Luzon Island (Alpert and General in prep.). These ants may be collected by leaf litter sifting, pitfall trapping and baiting with cookie crumbs on exposed rocks in creeks and rivers and following the forager to its nest in the rock’s crevices. Key to species: Seifert (2003) (Selected species-groups only).
Full-face and profile images of Philippine ant genera. Cardiocondyla sima, queen A, B Carebara alperti Fernandez, 2010 C, D Cataulacus chapmani Bolton, 1974 E, F.
Full-face and profile images of Philippine ant genera. Cardiocondyla sima, queen A, B Carebara alperti Fernandez, 2010 C, D Cataulacus chapmani Bolton, 1974 E, F.
Myrmicinae: 175 spp., 3 known from PH.
Figs. 13 C, D
The Philippine species of this pantropical genus are poorly known. There are specimens of two unidentified species from a transect study at Mt. Isarog, Bicol Region, Luzon Island (Alpert and General in prep.). Other species have been collected on Samar Island and in Misamis Occidental Province, Mindanao Island. These are very tiny ants with dimorphic workers. The major workers may possess a pair of tubercles or horns near the posterior margin of the head. These ants are ground-dwelling and may be collected by sifting soil and leaf litter. Key to species: Fernandez (2004) (New World only), Fernandez (2010) (description of first Philippine species).
Genus Cataulacus F. Smith, 1853Myrmicinae: 69 spp., 3 known from PH.
Figs. 13 E, F
These robust, hard-bodied ants have a wide, sculptured head and the antennal scrobe passing below the eye. They are known to be arboreal, can glide back to the tree trunk when they fall (Yanoviak et al. 2008) and nest in small hollow twigs or rotten branches of live trees. These ants may be collected by searching dead branches still attached to the tree and beating low vegetation over a white sheet. Key to species: Bolton (1974) (World revision).
Genus Centromyrmex Mayr, 1866bPonerinae: 15 spp., 1 known from PH.
Figs. 14 A, B
Centromyrmex feae (Emery, 1889) is the only species recorded from the Philippines at present. These small, cryptic ants have a pronotum that is somewhat flattened dorsally, mandibles that sharply curve downward and backward (in side view), and middle tibiae with strong peg-like setae. The workers are weakly polymorphic, with slight differences among nestmates. They are hypogaeic. Some evidence indicates that they are associated with termites. These ants may be collected by sifting leaf litter and soil cores at least 10 cm deep.
Full-face and profile images of Philippine ant genera. Centromyrmex feae A, B Cerapachys rufithorax Wheeler and Chapman, 1925 C, D Chronoxenus species PH01 E, F.
Full-face and profile images of Philippine ant genera. Centromyrmex feae A, B Cerapachys rufithorax Wheeler and Chapman, 1925 C, D Chronoxenus species PH01 E, F.
Cerapachyinae: 144 spp., 11 known from PH.
Figs. 14 C, D
These hard-bodied ants have a large, globular petiole and a larger, barrel-shaped postpetiole. They are predators on other ants and some species conduct group raids on other ant nests. During a raid, they steal the larvae and pupae which they sting. These stung prey remain alive for a long time, providing fresh food for the colony. In Mt. Isarog, Bicol Region, Luzon Island, they have been found nesting in twigs, with a larder of pupae of another ant genus, Crematogaster (Alpert and General in prep.). These ants may be collected by breaking open twigs on the ground and in the leaf litter, sifting leaf litter, and searching for columns of raiding workers. Key to species: Brown (1975) (World).
Genus Chronoxenus Santschi, 1919Dolichoderinae: 46 spp., 2 from PH.
Figs. 14 E, F
Dubovikov (2005) recently revived this genus from synonymy to receive all Oriental species of Bothriomyrmex Emery, 1869b. A specimen of an unidentified species, collected from Palawan Island in 1925, is in the ant collection of the Museum of Comparative Zoology, Harvard University. There are specimens of an undetermined species, collected in Laguna Province, Luzon Island, in the Insect Collection of the International Rice Research Institute. An excellent nest series with all the castes of possibly another species was recently collected by Perry Buenavente from Mt. Diwata, Agusan del Sur Province, Mindanao Island. These small ants, superficially similar to Tapinoma, have a petiole with an erect scale; with short, indistinct palps and a propodeum with a short dorsal face and a long declivity. These ants may be collected by sifting leaf litter including the humus layer. Emery (1925) (out of date); Shattuck (1992c) (generic revision of subfamily).
Genus Crematogaster Lund, 1831Myrmicinae: 780 spp., 16 known from PH.
Figs. 15 A, B
These small ants have a characteristically heart-shaped gaster that can flex over the mesosoma. They are often associated with coccids and aphids, sometimes building carton or soil shelters over these sap-sucking insects. They may be hypogaeic, epigaeic or arboreal. Similar to Cataulacus, they can glide back to the tree trunk when they fall, earning the nickname “acrobat ants” (Yanoviak et al. 2008). These ants may be collected by breaking open carton or soil that is at the apices of young branches, sifting leaf litter and soil, inspecting plant roots, beating low vegetation over a white sheet, and baiting with peanut butter on tree trunks or cookie crumbs on the ground. Key: Hosoishi and Ogata (2009a) (subgenus Physocrema); Hosoishi and Ogata (2009b) (checklist of Asian species).
Full-face and profile images of Philippine ant genera. Crematogaster difformis F. Smith, 1857 A, B Dacetinops cirrosus Taylor, 1985 C, D Diacamma panayense Wheeler and Chapman, 1925 E, F.
Full-face and profile images of Philippine ant genera. Crematogaster difformis F. Smith, 1857 A, B Dacetinops cirrosus Taylor, 1985 C, D Diacamma panayense Wheeler and Chapman, 1925 E, F.
Myrmicinae: 7 spp., 1 known from PH.
Figs. 15 C, D
(New record). Dr. Herbert Zettel (Natural History Museum, Vienna, Austria) has a single specimen, tentatively identified as Dacetinops cirrosus, collected near Calbiga-a River, Mt. Pangasugan, Baybay, Leyte Island. These ants bear spongiform tissue on the ventral surfaces of the petiole, postpetiole, and the first gastral segment. They nest in rotten wood. These ants may be collected by sifting leaf litter and breaking into rotten twigs and wood on the forest floor. Key to species: Brown and Wilson (1957), Taylor (1985) (Papuasian).
Genus Diacamma Mayr, 1862Ponerinae: 44 spp., 5 known from PH.
Figs. 15 E, F
This genus is long overdue for revision. These ants are easily distinguished from other ponerines by the distinctive costate sculpturing that covers the head, mesosoma, and the petiole, which has 2 dorsal spines. The late Dr. W.L. Brown, Jr. believed that male characters may hold the key to producing stable species boundaries. These large black ants are ground-dwelling or arboreal and hunt singly for prey. Ants of this genus have a unique social structure in which the queen caste is absent and all workers have the potential to mate and lay eggs. Peeters and Higashi (1989) (reproductive dominance behavior).
Genus Dilobocondyla Santschi, 1910Myrmicinae: 11 spp., 3 known from PH.
Figs. 16 A, B
These small hard-bodied ants have the upper corners of the head drawn into broad points and have a distinctive barrel-shaped petiole. They are known to be arboreal but may forage on the ground as well. An unidentified species is reported from a transect study at Mt. Isarog, Camarines Sur Province, Luzon Island (Alpert and General in prep.). These ants may be collected by beating low vegetation over a white sheet and inspecting dead branches still attached to the tree for nests.
Full-face and profile images of Philippine ant genera. Dilobocondyla chapmani Wheeler, 1924 A, B Discothyrea clavicornis Emery, 1897a C, D Dolichoderus thoracicus (F. Smith, 1860a) E, F.
Full-face and profile images of Philippine ant genera. Dilobocondyla chapmani Wheeler, 1924 A, B Discothyrea clavicornis Emery, 1897a C, D Dolichoderus thoracicus (F. Smith, 1860a) E, F.
Proceratiinae: 32 spp., 5 known from PH.
Figs. 16 C, D
(New record). There are specimens of Discothyrea bryanti (Wheeler, 1917) and Discothyrea clavicornis (Shattuck, pers. comm.) from adjacent stations of a transect study at Mt. Isarog by Joanaviva Caceres-Plopenio in 2006. This is remarkable because few species of this genus are sympatric, much less on the same transect (Shattuck, pers. comm.). Another unidentified species was found from a different transect study at Mt. Isarog, Bicol Region, Luzon Island (Alpert and General in prep.). Specimens were collected at 550-700 meters above sea level. Two unidentified species were extracted from lowland forest leaf litter berlesate from Samar Island. Another unidentified species was collected from Palawan Island. These small cryptic ants have an extremely large apical antennal segment and the petiole broadly attached to the gaster. They are known to be predators of arthropod eggs. These ants may be collected by sifting leaf litter, pitfall trapping, and searching for nests under rocks. Brown (1958) (generic revision).
Genus Dolichoderus Lund, 1831Dolichoderinae: 148 spp, 3 known from PH.
Figs. 16 E, F
The most common species, Dolichoderus thoracicus, is the black, hard-bodied ant usually associated with the locally-popular fruit of the lanzones tree (Meliaceae: Lansium domesticum Corr.), and of the makopa tree (Myrtaceae: Syzygium samarangense (Blume) Merrill and Perry) tending the mealybugs (Hemiptera: Sternorrhyncha: Pseudococcidae) that are found in the fruit bunches (DMG, pers. obs.). This ant, which forages day and night, is also common in urban and highly disturbed areas. These ants are easy to collect, with newly-mated queens even venturing indoors at night (DMG, unpubl. notes).
Genus Echinopla F. Smith, 1857Formicinae: 26 spp., 5 known from PH.
Figs. 17 A, B
Specimens of two unidentified species were collected from low vegetation in a transect study in Mt. Isarog, Bicol Region, Luzon Island (Alpert and General in prep.). One of these unidentified species was collected by nocturnal beating of low vegetation. These hairy or fuzzy ants are unique in appearance and have hard bodies and petioles armed with teeth or denticles laterally. These ants may be collected by beating of low vegetation over a white sheet and inspecting of dead branches in the foliage and canopy.
Full-face and profile images of Philippine ant genera. Echinopla pallipes F. Smith, 1857 A, B Euprenolepis species PH01 C, D Forelophilus species PH01 E, F.
Full-face and profile images of Philippine ant genera. Echinopla pallipes F. Smith, 1857 A, B Euprenolepis species PH01 C, D Forelophilus species PH01 E, F.
Formicinae: 6 spp., 2 known from PH.
Figs. 17 C, D
Euprenolepis negrosensis (Wheeler, 1930b) is known from the Philippines. It is rarely collected, perhaps because its tiny eyes indicate a subterranean life-habit. An unidentified species is depicted in Figs. 17 A, B. These ants have strongly curved mandibles such that the apical tooth is directed posteriolaterally. They forage underground, or in the leaf litter and on the ground, possibly for mushrooms, as found by Witte and Maschwitz (2008) in Malaysia. These ants may be collected by sifting leaf litter, pitfall trapping, soil core sampling and possibly by baiting with edible mushrooms. Key to species: LaPolla (2009) (World revision).
Genus Forelophilus Kutter, 1931Formicinae: 3 spp., 3 known from PH.
Figs. 17 E, F
This genus was previously known only from the type species, Forelophilus overbecki Kutter, 1931, described from Java. Two widespread species are now known from the Philippines, Forelophilus stefanschoedli Zettel and Zimmerman, 2007 known from the islands of Luzon, Leyte, and Mindanao, and Forelophilus philippinensis Zettel and Zimmerman, 2007 known from the islands of Luzon, Bayagnan, and Mindanao (Zettel and Zimmerman 2007). An unidentified third species is shown in Figures 17 E, F. These ants may be collected by beating low vegetation over a white sheet and hand collecting from foliage.
Genus Gauromyrmex Menozzi, 1933Myrmicinae: 2 spp., 1 known from PH.
Figs. 18 A, B
(New record). Gauromyrmex acanthinus (Karavaiev, 1935) is known from the Philippines. A single identified specimen is deposited in the MCZ Ant Collection. These small ants have a petiole with dorsolateral corners produced into acute angles or denticles. They are arboreal and may be collected by baiting with tuna or honey and by inspecting dead branches of live trees. Diagnosis: Bolton (2003).
Full-face and profile images of Philippine ant genera. Gauromyrmex acanthina A, B Gesomyrmex luzonensis, major worker C, D Gnamptogenys chapmani Brown, 1958 E, F.
Full-face and profile images of Philippine ant genera. Gauromyrmex acanthina A, B Gesomyrmex luzonensis, major worker C, D Gnamptogenys chapmani Brown, 1958 E, F.
Formicinae: 7 spp., 1 known from PH.
Figs. 18 C, D
Gesomyrmex luzonensis (Wheeler, 1916) is known from the Philippines. This ant is rarely collected but widespread, having been collected in a bamboo grove in Los Baños, Laguna Province, Luzon Island and in a hardwood cavity nest in Cuernos de Negros, Negros Island. All members of this genus are presumed to be nocturnal and arboreal in habitat. These ants have large bean-shaped eyes and mandibles that look like pinking scissors. They are polymorphic, with major, media, and minor workers. Wheeler (1930c) quoted J.W. Chapman’s description of the habits of Gesomyrmex luzonensis. Chapman found that the minors conduct most of the foraging, are attracted to ripe bananas, and are very timid. Key to species: Cole (1949); see also Wheeler (1929a, 1929b, 1930a).
Genus Gnamptogenys Roger, 1863Ectatomminae: 134 spp., 11 known from RP.
Figs. 18 E, F
These hard-bodied ants are specialized predators of other ant species. At least one species, Gnamptogenys menadensis (Mayr, 1887), has mostly fertile laying workers instead of a queen (Gobin et al. 1998, Gobin et al. 1999). They may be ground-dwelling or arboreal, diurnal or nocturnal. These ants may be collected by breaking open rotten wood on the ground, inspecting cavities in living trees and dead branches, sifting leaf litter, and pitfall trapping. Key to species: Lattke (2004) (SE Asia and Australasia).
Genus Harpegnathos Jerdon, 1851Ponerinae: 11 spp., 5 known from PH.
Figs. 19 A, B
These large cryptic ants bear characteristic long pliers-like mandibles and large eyes. They are usually ground-dwelling and hunt prey such as crickets in the leaf litter. A specimen of an unidentified arboreal species was collected on Mt. Isarog, Bicol Region, Luzon Island (DMG, unpubl. notes). These ants may be collected by sifting leaf litter, searching on the ground, and pitfall trapping.
Full-face and profile images of Philippine ant genera. Harpegnathos venator Donisthorpe, 1937 A, B Hypoponera confinis (Roger, 1860) C, D Iridomyrmex species PH01 E, F.
Full-face and profile images of Philippine ant genera. Harpegnathos venator Donisthorpe, 1937 A, B Hypoponera confinis (Roger, 1860) C, D Iridomyrmex species PH01 E, F.
Ponerinae: 171 spp., 6 known from PH.
Figs. 19 C, D
The taxonomy of this large pantropical genus is currently chaotic. These small, cryptic ants are very similar to Pachycondyla, but lack a second simple spur on the hind tibia. They have a simple ventral process of the petiole, without a fenestra or posterior angles as in Ponera. These ants make small colonies in soil, rotten wood, and leaf litter. These ants may be collected by sifting leaf litter, beating low vegetation, pitfall trapping, and searching in rotten wood. There is no modern key to species, however, Barry Bolton is currently revising this genus (B. Bolton, pers. comm.).
Genus Iridomyrmex Mayr, 1862Dolichoderinae: 82 spp., 2 known from PH.
Figs. 19 E, F
These fast-moving ants may be confused with Dolichoderus but have a thinner cuticle and bear the characteristic wavy anterior clypeal margin. A specimen of Iridomyrmex anceps (Roger, 1863) was recently collected from Marinduque Island by Joanaviva Caceres-Plopenio (P. S. Ward, pers. comm). There is a specimen of one unidentified species, collected from Baguio, Benguet Province, Luzon Island, in the MCZ ant collection. Other unidentified specimens have been collected from the provinces of Pangasinan, Isabela, and Camarines Sur on Luzon Island, and the islands of Camiguin and Semirara. They are ground-dwelling and usually form large colonies. These ants may be collected by sifting leaf litter, pitfall trapping, and baiting on the ground. Key to species: Heterick and Shattuck (2011) (World revision).
Genus Lepisiota Santschi, 1926Formicinae: 131 spp., 2 known from PH.
Figs. 20 A, B
These small, yellow or brown arboreal ants are characterized with a distinct angulate propodeum. They have been collected in the islands of Luzon, Negros, and Palawan, but rarely. These ants may be collected by beating low vegetation over a white sheet and inspecting cavities in living wood and dead branches in the canopy.
Full-face and profile images of Philippine ant genera. Lepisiota chapmani Wheeler, 1935 A, B Leptanilla astylina Petersen, 1968 (line drawing reprinted from Entomologiske Meddelelser, with permission) (C). Leptogenys maxillosa (F. Smith, 1858) D, E.
Full-face and profile images of Philippine ant genera. Lepisiota chapmani Wheeler, 1935 A, B Leptanilla astylina Petersen, 1968 (line drawing reprinted from Entomologiske Meddelelser, with permission) (C). Leptogenys maxillosa (F. Smith, 1858) D, E.
Leptanillinae: 43 spp., 1 known from PH.
Fig. 20 C
Only Leptanilla astylina is known from the Philippines (Palawan). It was described from the male alate. These tiny, blind, hypogaeic ants have a slim, elongated body, fully exposed antennal socket and a swollen postpetiole. They are extremely difficult to collect, perhaps because they live deep in the soil. Soil cores 10 cm deep during transect studies at Mt. Isarog failed to turn up any leptanilline ants (Alpert and General in prep.). These ants may be collected by sifting soil cores up to 30 cm deep and underground baiting with centipede carcasses. Key to species: Baroni Urbani (1977b) (World), Ogata et al. (1995).
Genus Leptogenys Roger, 1861Ponerinae: 248 spp., 11 known from PH.
Figs. 20 D, E
These long-bodied and slender ants bear the characteristic clypeus extending forward to form a rounded triangle. They also exhibit army ant-like behavior with large raiding columns and are known to prey on termites. They usually have worker-like, or ergatoid, queens and nest in rotten wood, under rocks, and in the ground. These ants may be collected by sifting leaf litter, flipping over rocks, pitfall trapping, and searching for raiding columns. Key to species: Wilson (1958) (Melanesian and New Caledonia).
Genus Leptomyrmex Mayr, 1862Dolichoderinae: 41 spp., 1 known from PH.
Figs. 21 A, B
This genus occurs mostly in Australia, New Guinea, New Caledonia, and Aru Island, Indonesia. Leptomyrmex is unique in the subfamily in having an elongated head, mesosoma, and legs. In addition, most of the queens are wingless. A single specimen of Leptomyrmex fragilis (F. Smith, 1859) has been found in the Bernice P. Bishop Museum collection. The specimen was collected by L.W. Quate in 1959 in San Francisco, Agusan del Sur Province, Mindanao Island. Lucky and Ward (2010) consider this specimen a doubtful record, however it is validated by the many legitimate records of other insects in the Quate collection. These ants may be collected by sifting leaf litter, pitfall trapping, baiting on the ground, and searching for nests at the base of trees and in rotten wood. Key to species: Lucky and Ward (2010). Generic review: Shattuck (1992c).
Full-face and profile images of Philippine ant genera. Leptomyrmex fragilis A, B Liomyrmex gestroi C, D Lophomyrmex bedoti Emery, 1893b E, F.
Full-face and profile images of Philippine ant genera. Leptomyrmex fragilis A, B Liomyrmex gestroi C, D Lophomyrmex bedoti Emery, 1893b E, F.
Myrmicinae: 1 sp., 1 known from PH.
Figs. 21 C, D
This is a monotypic tropical genus, with only one widespread species. Liomyrmex gestroi (Emery, 1887b) is known from throughout Southeast Asia. In the Philippines, it has been recorded from the islands of Luzon, Mindanao and Negros. This small, blind species has a smooth, almost hairless, body and bear large ventral processes on the petiole and postpetiole. It is assumed to be a kleptoparasite of mound-building termites, having been found inside live termite mounds and in forest leaf litter with termites. It may be collected by sifting leaf litter, pitfall trapping, and breaking into termite mounds to find nests. Rigato and Bolton (2001) (World revision).
Genus Lophomyrmex Emery, 1892aMyrmicinae: 12 spp., 1 known from PH.
Figs. 21 E, F
Lophomyrmex bedoti was collected by general collecting in Palawan. These monomorphic ants are hunter/scavengers, preying on various arthropods on the forest floor. They nest near or at the base of trees and form persistent soil-walled trails (similar to the trails of Pheidologeton ants), which may also run underground. These ants may be collected by baiting with sugar or protein bait and searching for nests at the base of trees and for conspicuous trails on the forest floor. Key to species: Rigato (1994) (World revision), Bharti and Kumar (2012) key to bedoti species-group.
Genus Lordomyrma Emery, 1897aMyrmicinae: 20 spp., 4 known from PH.
Figs. 33 A, B
Taylor (2012) recently described four new species from the Philippines:one species from Leyte Island and three sympatric species from Mt. Isarog Natural Park, Luzon Island. A specimen of an unidentified species was collected in a transect study in Bulacan Province, Luzon Island. These small monomorphic ants are variable in morphology but usually have a prominently convex pronotum. They forage in the foliage and on the ground. Little is known of their biology. These ants may be collected by beating low vegetation, sifting leaf litter, flipping rocks and breaking into rotten wood for nests. Taylor (2012) (descriptions and images of Philippine species).
Genus Mayriella Forel, 1902cMyrmicinae: 7 spp, 1 known from PH.
Figs. 22 C, D
The species known from the Philippines, Mayriella transfuga Baroni Urbani, 1977a, is wide-ranging in the Asian tropics and was originally described from Nepal. Ants of this genus are tiny, hard-bodied ants with a clypeus that extends as two lobes over the mandibles. The head and mesosoma are usually heavily sculptured while the gaster is smooth. They forage on the ground and nest in rotten wood and under stones. These ants may be collected by sifting leaf litter, pitfall trapping, and flipping stones and breaking into rotten wood for nests. Key to species: Shattuck and Barnett (2007) (World revision).
Genus Meranoplus F. Smith, 1853Myrmicinae: 62 spp., 2 known from PH.
Figs. 22 E, F
Only one species, Meranoplus biliran Schödl, 1998, is known from Biliran Island. There are specimens of another, possibly undescribed, species from the nearby island of Samar (Figs. 22 E, F). These slow-moving ants have the characteristic shield-like upper surface of the mesosoma, which is actually an extension of the pronotum. When disturbed, they curl up and remain motionless, similar to Basiceros and Calyptomyrmex ants. They are ground-dwelling generalist scavengers or seed predators and forage on the ground an in the foliage. They may be collected by beating low vegetation, sifting leaf litter, pitfall trapping, baiting with tuna or honey on trees, and searching for a ring of seed hulls around the nests. Key to species: Schödl (1998) (Oriental).
Full-face and profile images of Philippine ant genera. Lordomyrma emarginata Taylor 2012 A, B Mayriella transfuga C, D Meranoplus species PH01 E, F.
Full-face and profile images of Philippine ant genera. Lordomyrma emarginata Taylor 2012 A, B Mayriella transfuga C, D Meranoplus species PH01 E, F.
Myrmicinae: 17 spp., 2 known from PH.
Figs. 23 A, B
The two Philippine species are known only from winged reproductives. An unidentified species is present in the Bernice P. Bishop Museum ant collection. These hard-bodied cryptic ants are commonly mistaken for ponerine ants because of their large, broadly attached postpetiole. They have deep antennal scrobes and the clypeus projects forward as a square lobe. They are often found feeding on hardwood termites in dead logs and are very rarely collected. These ants may be collected by breaking into the heartwood of hardwood logs that are suspended off the ground. Key to species: Wheeler (1919c) (World), Alpert (2007) (Madagascar).
Full-face and profile images of Philippine ant genera. Metapone gracilis Wheeler, 1935 A, B Monomorium species PH01 C, D Myopias lobosa Willey and Brown, 1983 E, F.
Full-face and profile images of Philippine ant genera. Metapone gracilis Wheeler, 1935 A, B Monomorium species PH01 C, D Myopias lobosa Willey and Brown, 1983 E, F.
Myrmicinae: 399 spp., 5 known from PH.
Figs. 23 C, D
These tiny, smooth and slender ants have a single strong seta on the anterior clypeal margin. This genus includes worldwide invasive species such as Monomorium pharaonis (Linnaeus, 1758) and Monomorium floricola (Jerdon, 1851). They are typically opportunistic predators and scavengers. Their ground-nests are usually marked by a small crater of excavated soil. They may be collected by sifting leaf litter and pitfall trapping and searching for their nests on the ground. Key to species: Heterick (2001) (Australian).
Genus Myopias Roger, 1861Ponerinae: 36 spp., 5 known from PH.
Figs. 23 E, F
These cryptic ants have eyes that are very close to the base of the mandibles, and usually have a clypeal extension visible in the large gap between the mandibles. They nest in rotten twigs and logs and forage in the leaf litter and on the ground. They are known to prey on springtails, millipedes, and even other ants. These ants may be collected by sifting leaf litter and breaking into rotten wood. Willey and Brown (1983) (Australasian).
Genus Myopopone Roger, 1861Amblyoponinae: 1 sp., 1 known from PH.
Figs. 24 A, B
The sole species, Myopopone castanea (F. Smith 1860b), is widespread in the Philippines. This large-headed species has the characteristic flattening of the antennal flagella. It nests in Zorapteran-stage rotten wood, apparently preying on beetle larvae. It may be collected by breaking open rotten logs that you can dig into with a garden trowel. Brown (1960) (World revision).
Full-face and profile images of Philippine ant genera. Myopopone castanea A, B Myrmecina species PH01 C, D Myrmicaria brunnea subcarinata (F. Smith, 1857)E, F.
Full-face and profile images of Philippine ant genera. Myopopone castanea A, B Myrmecina species PH01 C, D Myrmicaria brunnea subcarinata (F. Smith, 1857)E, F.
Myrmicinae: 37 spp., 3 known from PH.
Figs. 24 C, D
This genus is widespread throughout the Philippines. There are specimens of several unidentified species from different transect studies on Mt. Isarog, Bicol Region, Luzon Island (Samson et al. 1997, Caceres in prep., Alpert and General in prep.). One of these species is smooth and reddish-orange and can be mistaken as Pristomyrmex ants. There are also specimens of another species from transect studies in the provinces of Bulacan and Nueva Vizcaya, Luzon Island. Specimens of yet another unidentified species were collected in Palawan Island. These hard-bodied ants have the characteristic ventral ridge of the head, running from the back of the head to the base of the mandibles, and the distinctive barrel-shaped petiole. They nest in twigs and rotten wood or under rocks and forage in the leaf litter. These ants may be collected by sifting leaf litter, pitfall trapping, and flipping over rocks.
Genus Myrmicaria Saunders, 1842Myrmicinae: 67 spp., 3 known from PH.
Figs. 24 E, F
These relatively large ants bear the characteristic 7-segmented antennae and long anterior peduncle of the petiole. They form conspicuous columns, forage on the ground and in the foliage, and are locally dominant where they occur. They are fairly easy to collect by baiting with tuna or honey, pitfall trapping, beating low vegetation, and hand collecting. Santschi (1925) (African; out of date); Bakhtiar et al. (2009) (morphological and behavioral notes of Southeast Asian species).
Genus Myrmoteras Forel, 1893bFormicinae: 32 spp., 4 known from PH
Figs. 25 A, B
These small trap-jawed ants have long mandibles that look like ripsaws and have very large eyes. They nest and hunt soft-bodied arthropods in the leaf litter. These ants may be collected by inspecting dead leaves that stick together then sifting the leaf litter. Keys to species: Moffett (1985) (World revision), Agosti (1992) (World revision), Zettel and Sorger (2011) (Philippine species).
Full-face and profile images of Philippine ant genera. Myrmoteras wlliamsi Wheeler, 1919 A, B Mystrium camillae Emery, 1889 C, D Noonilla copiosa (line drawings reprinted from Entomologiske Meddelelser, with permission) E, F.
Amblyoponinae: 9 spp., 1 known from PH.
Figs. 25 C, D
These cryptic ants have long, linear mandibles, inserted at the sides of the head, with blunt ends and a snaggle-tooth arrangement. These ground-foraging ants usually have soil particles stuck to their body, providing perfect camouflage. When disturbed, adults lie motionless. They nest under rocks and in rotten wood. These ants may be collected by sifting leaf litter, flipping over rocks, and breaking into rotten logs. Brown (1960) (World revision), Bihn and Verhaagh (2007) (Indo-Australian, with tabular key).
Genus Noonilla Petersen, 1968Leptanillinae: 1 sp., 2 known from PH.
Figs. 25 E, F
Described from an alate male reproductive, collected in southern Palawan Island, Noonilla copiosa Petersen, 1968 is the only described species of this genus. Ogata et al. (1995) examined a male specimen from Misamis Oriental Province, Mindanao Island and considered this genus incerta sedis in the subfamily. Bolton (2003) excluded the genus from Formicidae. However, Marek Borowiec (pers. comm.), who is currently studying the subfamily, is convinced that Noonilla belongs to Leptanillinae. These ants may be collected by sifting soil cores up to 30 cm deep, underground baiting for workers, and Malaise trapping for alates. Ogata et al. 1995, Bolton (2003) (synopsis of Formicidae).
Genus Nylanderia Emery, 1906Formicinae: 133 spp., 3 known from PH.
Figs. 26 A, B
This genus was recently revived from synonymy by LaPolla et al. 2010. There are at least 3 unidentified species from a transect study of Mt. Isarog, Bicol Region, Luzon Island (Alpert and General in prep.), including a morphological mimic of Tapinoma melanocephalum. These ants typically forage in the foliage, on the ground and in the leaf litter. They feed on honeydew from scale insects and aphids, and scavenge dead insects. These ants may be collected by beating of low vegetation, sifting leaf litter, pitfall trapping, and hand collecting. LaPolla et al. 2010 (Generic key to Prenolepis genus-group).
Full-face and profile images of Philippine ant genera. Nylanderia species PH01 A, B Ochetellus glaber C, D Odontomachus simillimus F. Smith, 1858 E, F.
Full-face and profile images of Philippine ant genera. Nylanderia species PH01 A, B Ochetellus glaber C, D Odontomachus simillimus F. Smith, 1858 E, F.
Dolichoderinae: 10 spp., 1 known from PH.
Figs. 26 C, D
The only species known from the Philippines, Ochetellus glaber (Mayr, 1862), is widespread in the archipelago. These fast-moving ants have a concave anterior clypeal margin and a concave propodeum. They may be arboreal or ground-foraging, sometimes forming conspicuous columns. These ants may be collected by beating of low vegetation, sifting leaf litter, and pitfall trapping. Shattuck (1992a) (generic revision).
Genus Odontomachus Latreille, 1804Ponerinae: 65 spp., 11 known from PH.
Figs. 26 E, F
These large, big-headed, trap-jawed ants are commonly referred to as “hantik” in many Philippine dialects. They are aggressive, locally dominant and ground-dwelling but may also climb the foliage to hunt for prey. One species, Odontomachus malignus F. Smith, 1859, is known to nest in rock crevices that are regularly inundated during high tide, whence they emerge and forage for animals trapped in the intertidal zone. Ants of this genus may be collected by baiting with tuna, beating of low vegetation, pitfall trapping, and hand collecting. Keys to species: Brown (1976) (World), Sorger and Zettel (2011) (Philippine species).
Genus Odontoponera Mayr, 1862Ponerinae: 2 spp., 1 known from PH.
Figs. 27 A, B
Odontoponera denticulata (F. Smith, 1858) is widespread in the Philippines. This moderately large, hard-bodied species has large blunt teeth on the sides of the pronotum and a crenulate anterior clypeal margin. It nests under bare ground and hunts singly. The nest entrance is a simple hole just large enough for one worker to pass through. This ant may be collected by sifting leaf litter, pitfall trapping, and hand collecting. Wheeler and Chapman (1925), Creighton (1929) (out of date), Yamane (2009).
Full-face and profile images of Philippine ant genera. Odontoponera denticulata A, B Oecophylla smaragdina (Fabricius, 1775) C, D Overbeckia subclavata Viehmeyer, 1916a (N.B. This is a specimen from Singapore. The Philippine specimen has its head crushed on its right side and the mesosoma is damaged as well.) E, F.
Full-face and profile images of Philippine ant genera. Odontoponera denticulata A, B Oecophylla smaragdina (Fabricius, 1775) C, D Overbeckia subclavata Viehmeyer, 1916a (N.B. This is a specimen from Singapore. The Philippine specimen has its head crushed on its right side and the mesosoma is damaged as well.) E, F.
Formicinae: 2 spp., 1 known from PH.
Figs. 27 C, D
Oecophylla smaragdina is widespread in the Philippines. This large yellow-green species weaves silken nests among the leaves of mango (Anacardiaceae: Mangifera indica L.) and other trees. It is aggressive and dominant where they occur, effectively excluding other ant species in the trees and on the ground as well. A single colony may construct many nests among several trees, with the outer nests serving as defensive bivouacs for older workers and the inner nests containing the queen and brood. Ilokanos, many Southeast Asian tribes, and south Chinese tribes harvest the pupae as a delicacy. This species is usually found at elevations below 500 meters. It may be collected by searching for nests in the canopy and hand collecting from the tree trunks.
Genus Overbeckia Viehmeyer, 1916aFormicinae: 1 sp., 1 known from PH.
Figs. 27 E, F
(New record). There is only one valid species in this genus, Overbeckia subclavata. This species has an antenna that gradually widens to a relatively broad terminal segment, and has a mesosoma that is flat and pinched at the metapleural spiracles. It was previously known only from Singapore, but a specimen in the MCZ Ant Collection is labeled "Bu of Sci, PI" and was collected by a certain M. Ramos (probably Maximo Ramos, who collected botanical specimens for the Bureau of Science from 1904 to 1932) (Van Steenis-Kruseman 2006). “PI” is the abbreviation for Philippine Islands, the old name used during the American colonial period. Overbeckia subclavata is a very rare ant, and its rediscovery will be an important event in Philippine myrmecology. This ant species is assumed to be arboreal because of its relatively large compound eyes. It may be collected by beating low vegetation over a white sheet and inspecting dead branches of living trees.
Genus Pachycondyla F. Smith, 1858Ponerinae: 289 spp., 16 known from PH.
Figs. 28 A, B
This large genus is also in dire need of taxonomic revision. These are small or large ants with two tibial spurs, a large pectinate spur behind a small simple one. They are abundant in the leaf litter and on the ground, hunting singly. They may be collected by sifting leaf litter, pitfall trapping, and baiting with honey or tuna bait. The genus Cryptopone was recently synonymized under Pachycondyla by MacKay and MacKay (2010).
Full-face and profile images of Philippine ant genera. Pachycondyla claudata (Menozzi, 1926) A, B Paraparatrechina iridescens (Donisthorpe, 1942) C, D Paratopula macta Bolton, 1988 E, F.
Full-face and profile images of Philippine ant genera. Pachycondyla claudata (Menozzi, 1926) A, B Paraparatrechina iridescens (Donisthorpe, 1942) C, D Paratopula macta Bolton, 1988 E, F.
Formicinae: 28 spp, 2 known from PH.
Figs. 28 C, D
This genus was recently revived from synonymy by LaPolla et al. 2010, who split the genus Paratrechina into 3 separate genera. Among these 3 genera, only Paraparatrechina ants have a pair of erect setae on the propodeum and erect setae on the pronotum and mesonotum arranged in neat pairs. Paraparatrechina iridescens and an unidentified species are known from several locations on Luzon Island. These ants may be collected by sifting leaf litter, pitfall trapping, and baiting with honey or tuna bait. LaPolla et al. 2010 (Generic key to Prenolepis genus-group).
Genus Paratopula Wheeler, 1919aMyrmicinae: 10 spp., 3 known from PH.
Figs. 28 E, F
There are ten valid species in this Oriental and Indo-Australian genus, with three species known from the Philippines. These large arboreal ants have a pronotum with a flat dorsal outline. They are rare, and may be collected by beating low vegetation over a white sheet and inspecting tree hollows and dead branches in the canopy. Key to species: Bolton (1988) (World).
Genus Paratrechina Motschoulsky, 1863Formicinae: 1 sp., 1 known from PH.
Figs. 29 A, B
As revised by LaPolla et al. (2010), this genus now contains only one species, the invasive species, Paratrechina longicornis (Latreille, 1802), widespread throughout the Philippines. The species readily invade households, farms and other highly-disturbed areas, recruiting large numbers of nestmates to scavenge dead insects and small animals and even table scraps. It may be collected by sifting leaf litter, pitfall trapping, and baiting with honey or tuna bait. LaPolla et al. 2010 (Generic key to Prenolepis genus-group).
Full-face and profile images of Philippine ant genera. Paratrechina longicornis A, B Pheidole hortensis Forel, 1913, major worker C, D Pheidologeton maccus Wheeler, 1929, major worker E, F.
Full-face and profile images of Philippine ant genera. Paratrechina longicornis A, B Pheidole hortensis Forel, 1913, major worker C, D Pheidologeton maccus Wheeler, 1929, major worker E, F.
Myrmicinae: 1, 121 spp., 21 known from PH.
Figs. 29 C, D
There are specimens of several unidentified species from a transect study of Mt. Isarog, Bicol Region, Luzon Island (Alpert and General in prep.). There are also unidentified species from transect studies in Isabela Province, Luzon Island and the islands of Samar and Mindanao. These tiny to small ants have the following character states: dimorphic, with large-headed major workers possessing usually edentate mandibles; pronotum strongly humped; and antennal club usually 3-segmented. They are ground-dwelling and forage on the ground and in the leaf litter, and may be collected by sifting leaf litter, pitfall trapping, and baiting with cookie crumbs. Eguchi (2001a) (Asian), (2001b) (Bornean), (2003) (morphology of male genitalia), (2004) (revision of Pheidole fervens and Pheidole indica).
Genus Pheidologeton Mayr, 1862Myrmicinae: 49 spp., 5 spp. and 4 subspp. known from PH.
Figs. 29 E, F
This is another genus which needs taxonomic revision. These tiny to small ants form conspicuous raiding columns, often protected by low walls of soil. They are extremely polymorphic with a continuous series of intermediates. Superficially similar to Pheidole, they can be distinguished by their 2-segmented antennal club and their polymorphism. And unlike Pheidole, the major and supermajor workers join the foraging column. They are ground-dwelling, and may be collected by sifting leaf litter, pitfall trapping, baiting with honey or tuna bait, and hand collecting. Ettershank (1966).
Genus Philidris Shattuck, 1992aDolichoderinae: 16 spp., 2 known from PH.
Figs. 30 A, B
Philidris myrmecodiae (Emery, 1887) was reported from Mt. Isarog, Camarines Sur, Luzon Island by Samson et al. (1997). There are specimens of an unidentified species, collected from Zamboanga, Mindanao Island, in the MCZ Ant Collection. There are also specimens from a transect study conducted in Isabela, Luzon Island. An entire nest series of an unidentified species was recently collected from a small island off eastern Mindanao (DMG, unpubl. notes). These small polymorphic ants are superficially similar to Iridomyrmex but have their eyes very low on the head. They nest in rotten wood or carton above the ground and in swollen specialized plant structures called domatia, which workers defend vigorously (Shattuck and Barnett 2010). They may be collected by beating low vegetation over a white sheet and inspecting the swollen bases of epiphytic plants. Shattuck (1992a, 1992b).
Full-face and profile images of Philippine ant genera. Philidris species PH01 A, B Plagiolepis species PH01 C, D Platythyrea parallela (F. Smith, 1859) E, F.
Full-face and profile images of Philippine ant genera. Philidris species PH01 A, B Plagiolepis species PH01 C, D Platythyrea parallela (F. Smith, 1859) E, F.
Formicinae: 86 spp., 2 known from PH.
Figs. 30 C, D
(New record). There are specimens of an unidentified species from a transect study of Mt. Isarog, Bicol Region, Luzon Island (Alpert and General in prep.) and of other species from transect studies conducted by Perry Buenavente in the provinces of Isabela and Nueva Vizcaya, Luzon Island. These tiny, cryptic ants have 11-segmented antennae and long palps. They nest in the ground under rocks and in rotten wood and forage in the leaf litter, on the ground, and in the foliage. These ants may be collected by sifting leaf litter, pitfall trapping, and beating low vegetation over a white sheet. Brown (1973).
Genus Platythyrea Roger, 1863Ponerinae: 46 spp., 4 known from PH.
Figs. 30 E, F
There are 46 valid species, including three from amber, in this cosmotropical genus. There are four valid species known from the Philippines. These ants have a shagreened or dull body surface and two pectinate tibial spurs on the hind leg. They may be ground-dwelling or nesting in trees, and are often found hunting individually. They may be collected by sifting leaf litter, pitfall trapping, and hand collecting on tree trunks. Key to species: Brown (1975) (World).
Genus Polyrhachis F. Smith, 1857Formicinae: 603 spp., 75 sp. and 10 subspp. known from PH.
Figs. 31 A, B
This is the largest ant genus in the Philippines. There are specimens of several unidentified species from a transect study of Mt. Isarog, Bicol Region, Luzon Island (Alpert and General in prep.). These small to large ants have spines on the pronotum, mesonotum, propodeum, or petiole or a combination of locations. They may nest in the ground, rotten logs or tree hollows. Some species are also known as weaver ants because they build nests in the foliage from larval silk and chewed-up plant fibers. These ants may be collected by beating low vegetation over a white sheet, pitfall trapping, and inspecting tree hollows and dead branches in the canopy. Keys: Hung (1967), Dorow (1995), (World, subgenera); Bolton (1975), Kohout (1987) (sexspinosa species-group), Dorow and Kohout (1995), Kohout (1998), Kohout (2006a) (cryptoceroides species-group), (2006b) (parabiotica species-group). Natural history: Dorow et al. 1990.
Full-face and profile images of Philippine ant genera. Polyrhachis cyaniventris F. Smith, 1858 A, B Ponera oreas (Wheeler, 1933) C, D Prenolepis species PH01 E, F.
Full-face and profile images of Philippine ant genera. Polyrhachis cyaniventris F. Smith, 1858 A, B Ponera oreas (Wheeler, 1933) C, D Prenolepis species PH01 E, F.
Ponerinae: 55 spp., 2 known from PH.
Figs. 31 C, D
These tiny cryptic ants may be confused with Hypoponera or Pachycondyla, but have a fenestra or translucent window in the ventral petiolar process. They forage in the leaf litter and on the ground and nest in rotten wood and under rocks. These ants may be collected by sifting leaf litter, pitfall trapping, breaking into rotten wood, and flipping over rocks. Key to species: Taylor (1967) (World, revision).
Genus Prenolepis Mayr, 1861Formicinae: 25 spp., 1 known from PH.
Figs. 31 E, F
There are specimens of an unidentified species from a transect study of Mt. Isarog, Bicol Region, Luzon Island (Alpert and General in prep.). These ants have the following character states: very long antennal scapes, at least half the length extending beyond the back of the head; mandibles not strongly curved, so that the apical tooth points to the side; and mesosoma elongated and constricted at midlength. They are ground-foraging. These ants may be collected by sifting leaf litter and pitfall trapping. LaPolla et al. 2010 (Generic key to Prenolepis genus-group).
Genus Prionopelta Mayr, 1866aAmblyoponinae: 13 spp., 1 known from PH.
Figs. 32 A, B
Prionopelta kraepelini Forel, 1905 was collected from a botanical transect study on Samar Island. This species is widespread, also known from several locations on the islands of Luzon, Negros and Palawan. Specimens are deposited in the National Museum of the Philippines in Manila, the UPLB-MNH, and the MCZ Ant Collection. These tiny, cryptic ants have mandibles with only 3 teeth. The workers forage in leaf litter and on the ground. These ants may be collected by sifting leaf litter and pitfall trapping. Keys to species: Brown (1960) (Indo-Australian, Neotropical), Shattuck (2008a) (Indo-Pacific).
Full-face and profile images of Philippine ant genera. Prionopelta kraepelini A, B Pristomyrmex bicolor Emery, 1900 C, D Probolomyrmex dammermani Wheeler, 1928 E, F.
Full-face and profile images of Philippine ant genera. Prionopelta kraepelini A, B Pristomyrmex bicolor Emery, 1900 C, D Probolomyrmex dammermani Wheeler, 1928 E, F.
Myrmicinae: 56 spp., 18 known from PH.
Figs. 32 C, D
There are specimens of an unidentified species from a transect study of Mt. Isarog, Bicol Region, Luzon Island (Alpert and General in prep.). These small attractive ants have the following character states: mandibles that are twisted so that the edges oppose each other; and exposed antennal sockets. They nest in rotten wood on the ground or under rocks and forage in the leaf litter and on the ground. These ants may be collected by sifting leaf litter, pitfall trapping, breaking into rotten logs and flipping over rocks. Keys to species: Wang (2003) (World revision), Zettel (2006, 2007) (Philippine species).
Genus Probolomyrmex Mayr, 1901Proceratiinae: 16 spp., 2 known from PH.
Figs. 32 E, F
One species is known from Negros Island, Probolomyrmex dammermani.There are specimens of an unidentified species collected by Joanaviva Caceres-Plopenio from a transect study of Mt. Isarog, Bicol Region, Luzon Island (Eguchi, pers. comm.; DMG, unpubl. notes). These tiny, cryptic ants have no eyes and bear their thick antennae on a shelf projecting forward from the head. They forage in the leaf litter and presumably also in the soil. These are among the rarest ants in the world. These ants may be collected by sifting leaf litter. Status of genus: Brown (1975). Keys to species: Taylor (1965), Eguchi et al. 2006.
Genus Proceratium Roger, 1863Proceratiinae: 80 spp., 1 known from PH.
Figs. 33 A, B
The species known from the Philippines, Proceratium papuanum Emery, 1897a, was collected from a transect study of Mt. Isarog, Bicol Region, Luzon Island (Alpert and General in prep.). These tiny ants have the following character states: apical segment of the antenna not extremely large or bulbous; and the petiole narrowly attached to the gaster. The workers forage in the leaf litter and on the ground. They may be collected by pitfall trapping and sifting leaf litter. Key to species: Baroni Urbani and de Andrade (2003) (World revision, including fossils).
Full-face and profile images of Philippine ant genera. Proceratium papuanum A, B Pseudolasius typhlops, major worker C, D Recurvidris species PH01 E, F.
Full-face and profile images of Philippine ant genera. Proceratium papuanum A, B Pseudolasius typhlops, major worker C, D Recurvidris species PH01 E, F.
Formicinae: 64 spp., 2 known from PH.
Figs. 33 C, D
This genus can be found in all the Old World tropics except Madagascar. Pseudolasius typhlops Wheeler, 1935b is known only from the Philippines. There are historical specimens of a different, unidentified species in the ant collection of the UPLB-MNH, and at least one unidentified species reported by Samson et al. 1997. Other unidentified species were recently collected from other parts of Luzon and from Mindanao Island. These are tiny to small, yellow polymorphic ants. They nest in rotten wood on the ground or underground, where they tend root-feeding coccids. These ants may be collected by sifting leaf litter and soil cores and underground baiting.
Genus Recurvidris Bolton, 1992Myrmicinae: 9 spp., 2 known from PH.
Figs. 33 E, F
There are specimens of at least 2 unidentified species from a transect study in Mt. Isarog, Bicol Region, Luzon Island (Alpert and General in prep.). There are also unidentified specimens from Mindanao Island. Zettel (2008) recently described Recurvidris nigrans from Negros Island. These small, slender ants have propodeal spines that curve upward and forward. They are ground-dwelling and forage in the leaf litter, and may be collected by sifting leaf litter, underground baiting and pitfall trapping. Keys to species: Bolton (1992), Zettel (2008).
Genus Rhopalomastix Forel, 1900aMyrmicinae: 7 spp., 1 known from PH.
Figs. 34 A, B
(New record). There are specimens of an unidentified species from Dumaguete, Negros Island in the MCZ Ant Collection. This species has a longitudinally striate head and mesosoma, and a postpetiole broadly attached to the gaster. It nests and forages in tunnels under bark. These ants are rare and may be collected by searching under the bark of living trees (DMG, GDA, unpubl. notes). Wheeler (1929c) (Review of genus).
Full-face and profile images of Philippine ant genera. Rhopalomastix species PH01, queen A, B Rhoptromyrmex wroughtonii C, D Rhytidoponera species PH01 E, F.
Full-face and profile images of Philippine ant genera. Rhopalomastix species PH01, queen A, B Rhoptromyrmex wroughtonii C, D Rhytidoponera species PH01 E, F.
Myrmicinae: 10 spp., 1 known from PH.
Figs. 34 C, D
There is one specimen, from “Boguio” [=Baguio City, Benguet Province, Luzon Island], of Rhoptromyrmex wroughtonii Forel, 1902a in the MCZ Ant Collection. This species was recently found to be abundant by Joanaviva Caceres-Plopenio during a transect study of an abandoned farm on Mt. Isarog, Camarines Sur, Luzon Island and was also collected by Perry Buenavente from a transect study on Mt. Palali, Nueva Vizcaya, Luzon Island (DMG, unpubl. notes). These small ants share similar morphological features with Tetramorium spp., e.g., a sharp clypeal ridge in front of antennal sockets and a lamella at the tip of the sting, but have a heart-shaped head, a broader, convex clypeus, and a keel-like petiolar venter. They are known to form large, ground-dwelling colonies nesting in rotten logs or in the ground and may be collected by sifting leaf litter and pitfall trapping. Keys to species: Brown (1964) (World revision); Bolton (1976) (World tribal revision), (1986) (World revision).
Genus Rhytidoponera Mayr, 1862Ectatomminae: 105 spp., 3 known from PH.
Figs. 34 E, F
Brown (1958) reports one species from the Philippines, Rhytidoponera araneoides (Le Guillou, 1842). There are specimens of an unidentified species from “San Francisco, Agusan”, Mindanao Island, in the MCZ Ant Collection. These hard-bodied ants are superficially similar to Gnamptogenys spp. but have a tooth at the anterolateral edge of the pronotum, and a triangular petiole in lateral view. They are generalist predators or scavengers and nest in the ground under rocks but may also be arboreal, and may be collected by sifting leaf litter, pitfall trapping, beating low vegetation over a white sheet and inspecting tree hollows and dead branches in the canopy.
Genus Romblonella Wheeler, 1935aMyrmicinae: 9 spp., 1 known from PH.
Figs. 35 A, B
There is one valid species known from the Philippines, Romblonella opaca (F. Smith, 1861). It is a widespread species, having been collected in the far-apart islands of Romblon, Rapu-rapu, and Palawan (DMG, unpubl. notes). This species has a large, bulbous, sessile petiole with a tiny denticle on the lower surface. It seems to be tolerant of human disturbance because the Rapu-rapu specimen was collected on a concrete sidewalk. Ants of this genus are ground-dwelling and may be collected by sifting leaf litter, pitfall trapping, and hand collecting. Keys to species: Bolton (1976) (World revision), Taylor (1991a) (Australian).
Full-face and profile images of Philippine ant genera. Romblonella opaca A, B Simopone conradti Emery, 1899 (N.B. Not from the Philippines, the unique holotype of Simopone chapmani is badly broken and cannot help the student visualize this rare genus) C, D Solenopsis geminata minor worker E, F.
Full-face and profile images of Philippine ant genera. Romblonella opaca A, B Simopone conradti Emery, 1899 (N.B. Not from the Philippines, the unique holotype of Simopone chapmani is badly broken and cannot help the student visualize this rare genus) C, D Solenopsis geminata minor worker E, F.
Cerapachyinae: 16 spp., 2 known from PH.
Figs. 35 C, D
The unique holotype specimen of Simopone chapmani Taylor, 1966, is deposited in the MCZ Ant Collection. These small ants bear a long barrel-shaped petiole and no tibial spurs on the middle legs. They are often arboreal, and presumed to be predators on other ants, and may be collected by beating low vegetation over a white sheet and examining dead twigs or branches in the canopy.
Genus Solenopsis Westwood, 1840Myrmicinae: 285 spp., 2 known from PH.
Figs. 35 E, F
Ants of this genus exhibit two main lifeways: the fire ants live independently in familiar ant mounds while the tiny thief ants nest beside other ants or termites. The species known in the Philippines, Solenopsis geminata, also called fire ants, is a common pest in households and agricultural areas. It is highly polymorphic, with a wide range of sizes between major and minor workers. There are unidentified specimens of Solenopsis subgenus Diplorhoptrum, which are tiny, monomorphic ants, collected by Perry Buenavente from a transect studies on Mt. Palali, Nueva Vizcaya Province, Luzon Island and 2 mountains in Mindanao Island (DMG, unpubl. notes). There are also specimens of an unidentified species from a transect study of Mt. Isarog, Bicol Region, Luzon Island (Alpert and General in prep.). These ants may be collected by sifting leaf litter, pitfall trapping, and hand collecting. Key to species: Trager (1991) (World, geminata-group).
Genus Stigmatomma Roger, 1859Amblyoponinae: 66 spp., 3 known from PH.
Figs. 10 A, B
Yoshimura and Fisher (2012) recently revived the status of this genus from synonymy. Philippine ants formerly referable to Amblyopone Erichson, 1842 are now transferred to this genus. These cryptic ants have characteristic elongate mandibles and a denticulate anterior clypeal border. In these ants, the gaster is broadly attached to the petiole. They are found under the bark of logs, in rotten wood, leaf litter, or soil and are known to prey on centipedes and beetle larvae. Key to species (as Amblyopone): Taylor (1979) (Melanesia).
Genus Strumigenys F. Smith, 1860cMyrmicinae: 774 spp., 30 spp. and 1 subsp. known from PH.
Figs. 36 A, B
There are specimens of an unidentified species from a transect studies of Mt. Isarog, Bicol Region, Luzon Island (Alpert and General in prep., Caceres-Plopenio, unpubl. M.S. thesis). There are also specimens of unidentified species from Eastern Samar Province, Samar Island and Polillo Island. These tiny to small, cryptic ants have spongy lobes at the sides of the propodeum, petiole, and postpetiole. They hunt for soft-bodied arthropod prey, usually Collembola (springtails), and nest in the leaf litter. These ants may be collected by inspecting rotten woody debris and dead leaves that are stuck together for nests before sifting leaf litter. Key to species: Bolton (2000) (World revision), Baroni Urbani and de Andrade 2007 (synonymy of Pyramica under Strumigenys).
Genus Tapinoma Foerster, 1850Dolichoderinae: 93 spp., 4 known from PH.
Figs. 36 C, D
The species known from the Philippines include the widespread invasive species, Tapinoma melanocephalum (Fabricius, 1793), which commonly invades households in search of sweet food. These small ants have a small, flat petiole and reflexed apex of the gaster, such that only four segments are visible in dorsal view. They may forage in the foliage or on the ground for dead insects but also tend coccids and aphids for honeydew. They nest in just about any available cavity in soil, under stones and bark, in living plants, and houses, and may be collected by sifting leaf litter, beating low vegetation over a white sheet, and baiting with sugar. Shattuck (1999), Fisher and Bolton (2007).
Full-face and profile images of Philippine ant genera. Strumigenys chapmani Brown, 1954 A, B Tapinoma williamsi C, D Technomyrmex albipes (F. Smith, 1861) E, F.
Full-face and profile images of Philippine ant genera. Strumigenys chapmani Brown, 1954 A, B Tapinoma williamsi C, D Technomyrmex albipes (F. Smith, 1861) E, F.
Dolichoderinae: 90 spp., 10 known from PH.
Figs. 36 E, F
The species known from the Philippines include the widespread invasive species, Technomyrmex albipes (F. Smith, 1861), which is very common in highly disturbed or agricultural areas. These ants also have a small, flat petiole but with five visible gastral segments in dorsal view. It can be difficult to distinguish them from Tapinoma, especially in shrunken or deformed specimens. They are scavengers and forage in the foliage and on the ground. They nest in the soil, in rotten wood, and under bark and rocks. These ants may be collected by sifting leaf litter, pitfall trapping, beating low vegetation over a white sheet, hand collecting, and flipping over rocks and loose bark. Key to species: Bolton (2007) (World revision).
Genus Tetramorium Mayr, 1855Myrmicinae: 459 spp., 28 from PH.
Figs. 37 A, B
The Philippine species include widespread invasive species such as Tetramorium lanuginosum Mayr, 1870, which are common in highly disturbed or agricultural areas (Wetterer, 2010). Schlick-Steiner et al. 2006 revived Tetramorium manobo (Calilung, 2000) from synonymy. There are specimens of several undescribed species (including a species misidentified as Leptothorax by Samson et al. 1997) from a transect study on Mt. Isarog, Bicol Region, Luzon Island (Alpert and General, in prep.). These small ants have a sharp clypeal ridge in front of the antennal sockets and a lamella at the tip of the sting, but the head is roughly rectangular in shape and with a straight-edged or slightly concave anterior clypeal margin. They are variable in size, sculpture of the cuticle, and color. These ants are generalist predators and scavengers, foraging on the ground, and nest in twigs, rotten wood, under rocks or bark. They may be collected by sifting leaf litter, pitfall trapping, breaking into rotten wood and twigs, and flipping over rocks and loose bark. Keys to species: Bolton (1976, 1977) (Oriental, Indo-Australian), Schlick-Steiner et al. 2006.
Full-face and profile images of Philippine ant genera. Tetramorium khnum Bolton, 1977 A, B Tetraponera allaborans (Walker, 1859) C, D Tyrannomyrmex species PH01 E, F.
Full-face and profile images of Philippine ant genera. Tetramorium khnum Bolton, 1977 A, B Tetraponera allaborans (Walker, 1859) C, D Tyrannomyrmex species PH01 E, F.
Pseudomyrmicinae: 118 spp., 9 known from PH.
Figs. 37 C, D
These long and slender arboreal ants have large, somewhat flattened eyes, and a reduced clypeus, such that the antennal sockets are near the front edge of the head. They nest in dead twigs and branches. These ants may be collected by beating low vegetation over a white sheet and inspecting dead twigs and branches in the canopy. Key to species: Ward (2001) (Oriental, Australian).
Genus Tyrannomyrmex Fernandez, 2003Myrmicinae: 3 sp., 1 known from PH.
Putative male alate: Figs. 37 E, F
This is a genus that is very rarely collected. The three known species were all described from unique worker specimens. A single male specimen, in the Philippine collection of the Bernice P. Bishop Museum of Hawaii, appears to belong to this genus because its mandible has only the apical and subapical teeth followed by a wide toothless masticatory margin and its curved petiole lacks a distinct node. If confirmed, it will be the first known male specimen of Tyrannomyrmex. These small ants have a curved petiole surmounted by a low node, and strongly-curved mandibles with only two teeth. They likely forage deep in the soil and only occasionally emerge in the leaf litter. These ants may be collected by underground baiting, sifting leaf litter and pitfall trapping. Fernandez (2003), Bolton et al. 2006.
Genus Vollenhovia Mayr, 1865Myrmicinae: 72 spp., 6 known from PH.
Figs. 38 A, B
These flat and slender ants have a large, keel-like ventral petiolar process. They nest in rotten wood and under rocks and forage in leaf litter. Little is known about their biology. They may be collected by sifting leaf litter, pitfall trapping, breaking into rotten wood and flipping over rocks. Bolton (2003).
Full-face and profile images of Philippine ant genera. Vollenhovia ambitiosa Menozzi, 1925 A, B Vombisidris philippina Zettel and Sorger, 2010 (image reproduced with permission of NHMW and antbase.net) C, D Unnamed genus PH01 E, F.
Full-face and profile images of Philippine ant genera. Vollenhovia ambitiosa Menozzi, 1925 A, B Vombisidris philippina Zettel and Sorger, 2010 (image reproduced with permission of NHMW and antbase.net) C, D Unnamed genus PH01 E, F.
Myrmicinae: 16 spp., 2 known from PH.
Figs. 38 C, D
A recently described species, V, was collected from five locations on three different islands, representing the first record of this genus in the Philippines (Zettel and Sorger 2010a). They also report at least another species, represented by a dealate queen from yet another location. These small ants are recognizable by a sinuate subocular groove running from the mandibular insertion to the latero-occipital margin of the head. Ants of this genus are arboreal, at least one species (Vombisidris humboldticola Zacharias & Rajan, 2004 from southern India) nesting in domatia, which are specialized swollen plant structures for harboring ants. These ants may be collected by beating low vegetation over a white sheet and inspecting domatia of epiphytes. Zettel and Sorger (2010a).
Unnamed genus PH01Myrmicinae: 1 sp., 1 known from PH.
Figs. 38 E, F
(New record). Specimens were extracted from berlesate from three locations on Samar Island. In Bolton (1994), this ant keys out to Mayriella, but bears little resemblance to it. The ant more closely resembles Tetheamyrma with its bidentate clypeal projection (S. Cover, pers. comm.), but has 10-segmented antennae and has no spatulate or lamellate hairs on the inner margin of the mandibles. Images are available online at: http://pick4.pick.uga.edu/mp/20q?act=x_antandpath=Insecta/Hymenoptera/Formicidae/Adelomyrmex/sp_phi1andname=Adelomyrmex+sp_phi1andxml=Ants_Philippinesandauthority=unknown+species. It may be collected by sifting leaf litter and pitfall trapping.
Unnamed genus PH02Myrmicinae: 1 sp. (?), 1 known from PH.
Figs. 39 A, B
(New record). There is a single specimen of this undescribed genus in the Philippine collection of the Bernice P. Bishop Museum in Honolulu, Hawaii. The collection was loaned to GDA, who found this unique specimen. The specimen was collected by L.W. Quate from San Francisco, Agusan del Sur in 1959. In Bolton (1994), this specimen keys out to “Undescribed genus” (p. 89) (P. Ward and S. Cover, pers.comm.). The diagnostic characteristic of this genus is the presence of the petiolar spiracle in the node, rather than in the peduncle. No collection information is included with the specimen, however, applying different collecting techniques may discover this species.
Full-face and profile images of Philippine ant genera. Unnamed genus PH02 A, B Unnamed genus PH03 C, D.
Full-face and profile images of Philippine ant genera. Unnamed genus PH02 A, B Unnamed genus PH03 C, D.
Myrmicinae: 2 spp., 2 known from PH.
Figs. 39 C, D
(New record). A good nest series of this undescribed genus was collected by Perry Buenavente in a transect study of Mt. Palali, Nueva Vizcaya Province, Luzon Island. A unique specimen of another species (now lost due to theft, Taylor, pers. comm.) was earlier collected by Bambet Alto from Mt. Isarog. These reddish-orange ants seem to prefer high elevations, having been collected from >1450 masl at both locations. These ants may nest in rotten wood (DMG, unpubl. notes). They are characterized by stemmed mandibles with blunt or rounded denticles and a subsessile petiole with an angle protecting the spiracle. These ants may be collected by breaking into rotten wood, sifting leaf litter and pitfall trapping.
Key to the subfamilies of Philippine ants, based on the worker caste (adapted from Bolton 1994)* Numbers in parentheses refer to the previous couplet in the sequence
| 1 | Postpetiole absent | 2 |
| – | Postpetiole present and petiole and pospetiole nearly equal in size, or postpetiole much larger, in which case, gaster with distinct constriction at insertion with postpetiole | 7 |
| 2(1) | Sting absent | 3 |
| – | Sting present, often conspicuous and functional | 4 |
| 3(2) | In profile, a circular nozzle, the acidopore, often fringed with hairs, present at apex of gaster; antennal sockets sometimes situated well behind posterior margin of clypeus | Formicinae |
| – | In profile, acidopore absent; apex of gaster with a transverse slit-like orifice (best seen in apical or lateral view); antennal sockets always abutting the posterior margin of clypeus | Dolichoderinae |
| 4(2) | Petiole broadly attached to gaster, so that petiole has no posterior face in side view | Amblyoponinae |
| – | Petiole narrowly attached to gaster, so that petiole has a posterior face in side view | 5 |
| 5(4) | Frontal lobes and clypeus absent or reduced, so that antennal sockets are fully exposed in dorsal view and situated very near or above anterior margin of the head | Proceratiinae |
| – | Frontal lobes and clypeus well-developed, so that antennal sockets are partially or entirely covered in dorsal view and situated posterior to the anterior margin of the head | 6 |
| 6(5) | Frontal lobes elongate and roughly parallel, so that frontal carinae do not converge posteriorly; mandibles triangular | Ectatomminae |
| – | Frontal lobes rounded or bluntly triangular; frontal carinae, when present, appear to converge posteriorly; mandibles variable from linear to triangular | Ponerinae |
| 7(1) | Pronotum and mesonotum separated by a conspicuous, flexible suture, allowing pronotum to move relative to mesonotum | 8 |
| – | Pronotum and mesonotum separated by an often inconspicuous, rigid suture, so that pronotum and mesonotum are fused together | 9 |
| 8(7) | Eyes, present, large; ventral surface of postpetiole at most slightly bulging (Tetraponera) (Figs. 37 C, D) | Pseudomyrmecinae |
| – | Eyes absent; ventral surface of postpetiole usually swollen and bulbous | Leptanillinae |
| 9(7) | Postpetiole barrel-shaped and much larger than petiole; gaster conspicuously constricted at insertion; upper surface of tip of gaster transversely flattened and armed with peg-like teeth | Cerapachyinae |
| – | Postpetiole globular and nearly equal in size to petiole; gaster with narrow constriction at insertion; upper surface of tip of gaster rounded and unarmed | 10 |
| 10(9) | With all the following character states: frontal lobes absent; antennal sockets completely exposed; eyes absent; gaster narrowly constricted at insertion with postpetiole (Aenictus) (Figs. 9 E, F) | Aenictinae |
| – | Not as above: either frontal lobes present (much reduced or absent in Pristomyrmex), antennal sockets partially or completely concealed (except in Pristomyrmex); eyes present, or gaster not constricted at insertion with postpetiole | Myrmicinae |
| 1 | Postpetiole absent | 2 |
| – | Postpetiole present and petiole and pospetiole nearly equal in size, or postpetiole much larger, in which case, gaster with distinct constriction at insertion with postpetiole | 7 |
| 2(1) | Sting absent | 3 |
| – | Sting present, often conspicuous and functional | 4 |
| 3(2) | In profile, a circular nozzle, the acidopore, often fringed with hairs, present at apex of gaster; antennal sockets sometimes situated well behind posterior margin of clypeus | Formicinae |
| – | In profile, acidopore absent; apex of gaster with a transverse slit-like orifice (best seen in apical or lateral view); antennal sockets always abutting the posterior margin of clypeus | Dolichoderinae |
| 4(2) | Petiole broadly attached to gaster, so that petiole has no posterior face in side view | Amblyoponinae |
| – | Petiole narrowly attached to gaster, so that petiole has a posterior face in side view | 5 |
| 5(4) | Frontal lobes and clypeus absent or reduced, so that antennal sockets are fully exposed in dorsal view and situated very near or above anterior margin of the head | Proceratiinae |
| – | Frontal lobes and clypeus well-developed, so that antennal sockets are partially or entirely covered in dorsal view and situated posterior to the anterior margin of the head | 6 |
| 6(5) | Frontal lobes elongate and roughly parallel, so that frontal carinae do not converge posteriorly; mandibles triangular | Ectatomminae |
| – | Frontal lobes rounded or bluntly triangular; frontal carinae, when present, appear to converge posteriorly; mandibles variable from linear to triangular | Ponerinae |
| 7(1) | Pronotum and mesonotum separated by a conspicuous, flexible suture, allowing pronotum to move relative to mesonotum | 8 |
| – | Pronotum and mesonotum separated by an often inconspicuous, rigid suture, so that pronotum and mesonotum are fused together | 9 |
| 8(7) | Eyes, present, large; ventral surface of postpetiole at most slightly bulging (Tetraponera) (Figs. 37 C, D) | Pseudomyrmecinae |
| – | Eyes absent; ventral surface of postpetiole usually swollen and bulbous | Leptanillinae |
| 9(7) | Postpetiole barrel-shaped and much larger than petiole; gaster conspicuously constricted at insertion; upper surface of tip of gaster transversely flattened and armed with peg-like teeth | Cerapachyinae |
| – | Postpetiole globular and nearly equal in size to petiole; gaster with narrow constriction at insertion; upper surface of tip of gaster rounded and unarmed | 10 |
| 10(9) | With all the following character states: frontal lobes absent; antennal sockets completely exposed; eyes absent; gaster narrowly constricted at insertion with postpetiole (Aenictus) (Figs. 9 E, F) | Aenictinae |
| – | Not as above: either frontal lobes present (much reduced or absent in Pristomyrmex), antennal sockets partially or completely concealed (except in Pristomyrmex); eyes present, or gaster not constricted at insertion with postpetiole | Myrmicinae |
| 1 | Frontal lobes large, extending forward beyond anterior clypeal margin; antennal funiculus flattened or compressed (Figs. 24 A, B) | Myopopone |
| – | Frontal lobes never extending beyond anterior clypeal margin; antennal funiculus more or less circular in cross-section | 2 |
| 2(1) | Mandibles with only 3 teeth, with median tooth smallest; at full closure, little or no gap between mandibles and anterior clypeal margin (Figs. 32 A, B) | Prionopelta |
| – | Mandibles with more than 3 teeth; at full closure, large gap between mandibles and anterior clypeal margin | 3 |
| 3(2) | Posterior margin of head strongly concave; apex of mandible blunt or rounded (Figs. 25 C, D) | Mystrium |
| – | Posterior margin of head at most weakly concave; apex of mandible acute (Figs. 10 A, B) | Stigmatomma |
| 1 | Frontal lobes large, extending forward beyond anterior clypeal margin; antennal funiculus flattened or compressed (Figs. 24 A, B) | Myopopone |
| – | Frontal lobes never extending beyond anterior clypeal margin; antennal funiculus more or less circular in cross-section | 2 |
| 2(1) | Mandibles with only 3 teeth, with median tooth smallest; at full closure, little or no gap between mandibles and anterior clypeal margin (Figs. 32 A, B) | Prionopelta |
| – | Mandibles with more than 3 teeth; at full closure, large gap between mandibles and anterior clypeal margin | 3 |
| 3(2) | Posterior margin of head strongly concave; apex of mandible blunt or rounded (Figs. 25 C, D) | Mystrium |
| – | Posterior margin of head at most weakly concave; apex of mandible acute (Figs. 10 A, B) | Stigmatomma |
| 1 | Antennal sockets set close together, separated by a narrow triangular posterior extension of the clypeus; mesotibial spurs present (Figs. 14 C, D) | Cerapachys |
| – | Antennal sockets widely separated, set apart by a wide triangular posterior extension of the clypeus; mesotibial spurs absent (Figs. 35 C, D) | Simopone |
| 1 | Antennal sockets set close together, separated by a narrow triangular posterior extension of the clypeus; mesotibial spurs present (Figs. 14 C, D) | Cerapachys |
| – | Antennal sockets widely separated, set apart by a wide triangular posterior extension of the clypeus; mesotibial spurs absent (Figs. 35 C, D) | Simopone |
| 1 | In profile, petiole overhung by anterior part of gaster and node absent | 2 |
| – | In profile, petiole with a conspicuous node, and not overhung by gaster | 3 |
| 2(1) | In profile, gaster with 4 dorsal segments (tergites), the fifth segment reflexed so that anal pore is ventral, not a termination of gaster (Figs. 36 C, D) | Tapinoma |
| – | In profile, gaster with 5 dorsal segments (tergites) and anal pore is terminal (Figs. 36 E, F) | Technomyrmex |
| 3(1) | Palps short and hard to see; in profile, dorsal face of propodeum much shorter than propodeal declivity, giving the mesosoma a compact appearance (Figs. 14 E, F) | Chronoxenus |
| – | Palps long and conspicuous; dorsal face and declivity of propodeum about equal in length so that the mesosoma appears elongated | 4 |
| 4(3) | Head and mesosoma much longer than broad; legs extremely elongated (Figs. 21 A, B) | Leptomyrmex |
| – | Head roughly triangular and mesosoma not elongated; legs not elongated | 5 |
| 5(4) | Mesosoma with thick and sculptured integument (Figs. 16 E, F) | Dolichoderus |
| – | Mesosoma with thin integument and generally smooth or shagreened | 6 |
| 6(5) | In profile, rear face of propodeum concave; in side view, metanotal groove a narrow, distinct notch (Figs. 26 C, D) | Ochetellus |
| – | In profile, rear face of propodeum convex, rarely flat; in side view, metanotal groove a broad impression | 7 |
| 7(6) | In frontal view, posterior margin of head strongly concave and eyes located anterior to midline of of head; petiolar node inclined forward; polymorphic workers (Figs. 30 A, B) | Philidris |
| – | In frontal view, posterior margin of head at most weakly concave, usually flat or convex; eyes posterior to midline of head; petiolar node more or less vertical; monomorphic workers (Figs. 19 E, F) | Iridomyrmex |
| 1 | In profile, petiole overhung by anterior part of gaster and node absent | 2 |
| – | In profile, petiole with a conspicuous node, and not overhung by gaster | 3 |
| 2(1) | In profile, gaster with 4 dorsal segments (tergites), the fifth segment reflexed so that anal pore is ventral, not a termination of gaster (Figs. 36 C, D) | Tapinoma |
| – | In profile, gaster with 5 dorsal segments (tergites) and anal pore is terminal (Figs. 36 E, F) | Technomyrmex |
| 3(1) | Palps short and hard to see; in profile, dorsal face of propodeum much shorter than propodeal declivity, giving the mesosoma a compact appearance (Figs. 14 E, F) | Chronoxenus |
| – | Palps long and conspicuous; dorsal face and declivity of propodeum about equal in length so that the mesosoma appears elongated | 4 |
| 4(3) | Head and mesosoma much longer than broad; legs extremely elongated (Figs. 21 A, B) | Leptomyrmex |
| – | Head roughly triangular and mesosoma not elongated; legs not elongated | 5 |
| 5(4) | Mesosoma with thick and sculptured integument (Figs. 16 E, F) | Dolichoderus |
| – | Mesosoma with thin integument and generally smooth or shagreened | 6 |
| 6(5) | In profile, rear face of propodeum concave; in side view, metanotal groove a narrow, distinct notch (Figs. 26 C, D) | Ochetellus |
| – | In profile, rear face of propodeum convex, rarely flat; in side view, metanotal groove a broad impression | 7 |
| 7(6) | In frontal view, posterior margin of head strongly concave and eyes located anterior to midline of of head; petiolar node inclined forward; polymorphic workers (Figs. 30 A, B) | Philidris |
| – | In frontal view, posterior margin of head at most weakly concave, usually flat or convex; eyes posterior to midline of head; petiolar node more or less vertical; monomorphic workers (Figs. 19 E, F) | Iridomyrmex |
| 1 | In profile, anteroventral margin of pronotum with distinct tooth; hind pretarsal claw with median tooth (Figs. 34 E, F) | Rhytidoponera |
| – | In profile, anteroventral margin of pronotum rounded or bluntly angulate; hind pretarsal claw without median tooth (Figs. 18 E, F) | Gnamptogenys |
| 1 | In profile, anteroventral margin of pronotum with distinct tooth; hind pretarsal claw with median tooth (Figs. 34 E, F) | Rhytidoponera |
| – | In profile, anteroventral margin of pronotum rounded or bluntly angulate; hind pretarsal claw without median tooth (Figs. 18 E, F) | Gnamptogenys |
| 1 | Antenna 8-segmented, folding back below eye; eyes always large (Figs. 18 C, D) | Gesomyrmex |
| – | Antenna with 9- to 12-segmented, folding back above eye; eyes variable in size | 2 |
| 2(1) | Antenna 9- to 11-segmented | 3 |
| – | Antenna 12-segmented | 6 |
| 3(2) | Propodeum and petiole armed with a pair of spines or teeth (Figs. 20 A, B) | Lepisiota |
| – | Propodeum and petiole without spines or teeth | 4 |
| 4(3) | Palps very short and extremely difficult to see; eyes minute (Figs. 9 C, D) | Acropyga |
| – | Palps long and prominent; eyes usually well-developed, often large | 5 |
| 5(4) | Antennal scapes extremely long, extending more than half their length beyond posterior margin of head; erect hairs absent on dorsum of mesosoma (Figs. 11 C, D) | Anoplolepis |
| – | Antennal scape, notably shorter, seldom extending much beyond posterior margin of head; erect hairs sometimes present on dorsum of mesosoma (Figs. 30 C, D) | Plagiolepis |
| 6(2) | Mandibles with 10 or more teeth or denticles | 7 |
| – | Mandibles with fewer than 10 teeth or denticles | 8 |
| 7(6) | Mandibles linear and longer than head length, with sharp teeth along most of their length and crossing at apices when closed; eyes enormous (Figs. 25 A, B) | Myrmoteras |
| – | Mandibles triangular and shorter than head length; eyes large but not taking up most of the sides of head (Figs. 27 C, D) | oecophylla |
| 8(6) | Antennal sockets almost abutting posterior clypeal margin; ring of hairs present around acidopore | 9 |
| – | Antennal sockets well posterior to posterior clypeal margin; ring of hairs often absent around acidopore | 15 |
| 9(8) | Maxillary palp short and inconspicuous, with 2–4 segments | 10 |
| – | Maxillary palp long and conspicuous, with 6 segments | 11 |
| 10(9) | In side view, mesonotal constriction present; mandibles strongly curved (Figs. 17 C, D) | Euprenolepis |
| – | In side view, mesonotal constriction absent; mandibles not strongly curved (Figs. 33 C, D) | Pseudolasius |
| 11(9) | Mesosoma and head without coarse erect hairs (Figs. 27 E, F) | Overbeckia |
| – | Mesosoma and head with coarse erect hairs | 12 |
| 12(11) | In side view, mesosoma long and slender, with or without constriction of mesonotum | 13 |
| – | In side view, mesosoma short and compact, without constriction of mesonotum | 14 |
| 13(12) | In side view, pronotum only slightly convex; erect setae on head randomly scattered on surface (Figs. 29 A, B) | Paratrechina |
| – | In side view, pronotum convex; erect setae on head form 2 parallel rows (Figs. 31 E, F) | Prenolepis |
| 14(12) | Propodeum with 1 pair of erect setae (Figs. 28 C, D) | Paraparatrechina |
| – | Propodeum without a pair of erect setae (Figs. 26 A, B) | Nylanderia |
| 15(8) | In side view, metathoracic spiracles forming turbercles that are the highest prominences of the mesosoma (Figs. 17 E, F) | Forelophilus |
| – | In side view, metathoracic spiracles not forming tubercles that are the highest prominences of the mesosoma | 16 |
| 16(15) | Petiole node lacking teeth or spines; first gastral tergite distinctly less than half total length of gaster (Figs. 12 E, F) | Camponotus |
| – | Petiole node armed with spines, teeth, or denticles; first gastral tergite large, at least half of total length of gaster | 17 |
| 17(16) | Spines or teeth usually present on pronotum, propodeum, or both; body usually covered with short appressed hairs and some erect hairs (Figs. 31 A, B) | Polyrhachis |
| – | Spines or teeth absent from pronotum and propodeum, often present on petiole; body usually densely covered with long erect hairs (Figs. 17 A, B) | Echinopla |
| 1 | Antenna 8-segmented, folding back below eye; eyes always large (Figs. 18 C, D) | Gesomyrmex |
| – | Antenna with 9- to 12-segmented, folding back above eye; eyes variable in size | 2 |
| 2(1) | Antenna 9- to 11-segmented | 3 |
| – | Antenna 12-segmented | 6 |
| 3(2) | Propodeum and petiole armed with a pair of spines or teeth (Figs. 20 A, B) | Lepisiota |
| – | Propodeum and petiole without spines or teeth | 4 |
| 4(3) | Palps very short and extremely difficult to see; eyes minute (Figs. 9 C, D) | Acropyga |
| – | Palps long and prominent; eyes usually well-developed, often large | 5 |
| 5(4) | Antennal scapes extremely long, extending more than half their length beyond posterior margin of head; erect hairs absent on dorsum of mesosoma (Figs. 11 C, D) | Anoplolepis |
| – | Antennal scape, notably shorter, seldom extending much beyond posterior margin of head; erect hairs sometimes present on dorsum of mesosoma (Figs. 30 C, D) | Plagiolepis |
| 6(2) | Mandibles with 10 or more teeth or denticles | 7 |
| – | Mandibles with fewer than 10 teeth or denticles | 8 |
| 7(6) | Mandibles linear and longer than head length, with sharp teeth along most of their length and crossing at apices when closed; eyes enormous (Figs. 25 A, B) | Myrmoteras |
| – | Mandibles triangular and shorter than head length; eyes large but not taking up most of the sides of head (Figs. 27 C, D) | oecophylla |
| 8(6) | Antennal sockets almost abutting posterior clypeal margin; ring of hairs present around acidopore | 9 |
| – | Antennal sockets well posterior to posterior clypeal margin; ring of hairs often absent around acidopore | 15 |
| 9(8) | Maxillary palp short and inconspicuous, with 2–4 segments | 10 |
| – | Maxillary palp long and conspicuous, with 6 segments | 11 |
| 10(9) | In side view, mesonotal constriction present; mandibles strongly curved (Figs. 17 C, D) | Euprenolepis |
| – | In side view, mesonotal constriction absent; mandibles not strongly curved (Figs. 33 C, D) | Pseudolasius |
| 11(9) | Mesosoma and head without coarse erect hairs (Figs. 27 E, F) | Overbeckia |
| – | Mesosoma and head with coarse erect hairs | 12 |
| 12(11) | In side view, mesosoma long and slender, with or without constriction of mesonotum | 13 |
| – | In side view, mesosoma short and compact, without constriction of mesonotum | 14 |
| 13(12) | In side view, pronotum only slightly convex; erect setae on head randomly scattered on surface (Figs. 29 A, B) | Paratrechina |
| – | In side view, pronotum convex; erect setae on head form 2 parallel rows (Figs. 31 E, F) | Prenolepis |
| 14(12) | Propodeum with 1 pair of erect setae (Figs. 28 C, D) | Paraparatrechina |
| – | Propodeum without a pair of erect setae (Figs. 26 A, B) | Nylanderia |
| 15(8) | In side view, metathoracic spiracles forming turbercles that are the highest prominences of the mesosoma (Figs. 17 E, F) | Forelophilus |
| – | In side view, metathoracic spiracles not forming tubercles that are the highest prominences of the mesosoma | 16 |
| 16(15) | Petiole node lacking teeth or spines; first gastral tergite distinctly less than half total length of gaster (Figs. 12 E, F) | Camponotus |
| – | Petiole node armed with spines, teeth, or denticles; first gastral tergite large, at least half of total length of gaster | 17 |
| 17(16) | Spines or teeth usually present on pronotum, propodeum, or both; body usually covered with short appressed hairs and some erect hairs (Figs. 31 A, B) | Polyrhachis |
| – | Spines or teeth absent from pronotum and propodeum, often present on petiole; body usually densely covered with long erect hairs (Figs. 17 A, B) | Echinopla |
| 1 | Metanotal groove absent (Fig. 20 C) | Leptanilla |
| – | Metanotal groove present and distinct | 2 |
| 2 | Petiole with a posterior face | Protanilla |
| – | Petiole without a posterior face (Figs. 11 A, B) | Anomalomyrma |
| 1 | Metanotal groove absent (Fig. 20 C) | Leptanilla |
| – | Metanotal groove present and distinct | 2 |
| 2 | Petiole with a posterior face | Protanilla |
| – | Petiole without a posterior face (Figs. 11 A, B) | Anomalomyrma |
| 1 | In side view, antennal scrobe present below eye | 2 |
| – | In side view, antennal scrobe absent or present above eye | 3 |
| 2(1) | Antenna 7-segmented; antennal scape triangular, widest near insertion and tapering distally; petiole pedunculate; propodeum armed with thin longitudinal flanges (Figs. 12 A, B) | Basiceros |
| – | Antenna 11-segmented; antennal scape rod-like; petiole sessile; propodeum armed with 2 thick spines (Figs. 13 E, F) | Cataulacus |
| 3(1) | Petiole lacking a distinct node | 4 |
| – | Petiole with a distinct node | 6 |
| 4(3) | Petiole transversely flattened; in dorsal view, in dorsal view, gaster roughly heart-shaped; (Figs. 15 A, B) | Crematogaster |
| – | Petiole roughly barrel-shaped; in dorsal view, gaster not heart-shaped | 5 |
| 5(4) | Side of head with a longitudinal ridge running below the eye, from rear border to mandibular insertion; posterolateral corner of the head not forming a blunt point (Figs. 24 C, D) | Myrmecina |
| – | Side of head lacking a ridge below the eye; posterolateral corner of the head forming a blunt point (Figs. 16 A, B) | Dilobocondyla |
| 6(3) | Mandibles bidentate (with 2 teeth); mesosoma without a metanotal groove (Figs. 37 E, F) | Tyrannomyrmex |
| – | Mandibles with more than 2 teeth; mesosoma usually with a metanotal groove | 7 |
| 7(6) | Petiole and postpetiole with at least some light-colored sponge-like tissue | 8 |
| – | Petiole and postpetiole never with sponge-like tissue | 9 |
| 8(7) | Sponge-like tissue only on ventral surface of petiole, postpetiole, and the 4th abdominal segment; antenna 11-segmented (Figs. 15 C, D) | Dacetinops |
| – | Sponge-like tissue on lateral, as well as ventral, surfaces of petiole, postpetiole, and 4th abdominal segment; antenna 4–to 6-segmented (Figs. 36 A, B) | Strumigenys |
| 9(7) | Antenna with a distal club of 2 segments | 10 |
| – | Antenna with a distal club of 3 or 4 segments, or with an indistinct club | 15 |
| 10(9) | Antennal scrobe present; head and mesosoma punctuate (Figs. 22 C, D) | Mayriella |
| – | Antennal scrobe absent; head and mesosoma smooth, rugose, or reticulate | 11 |
| 11(10) | Head and mesosoma sculptured; petiole at most with a short peduncle | 12 |
| – | Head and mesosoma smooth between sparse punctures; petiole pedunculate | 13 |
| 12(11) | Head and mesosoma longitudinally rugose; propodeum unarmed; gaster broadly attached to postpetiole (Figs. 34 A, B) | Rhopalomastix |
| – | Head and mesosoma strongly reticulate; propodeum bidentate; gaster narrowly attached to postpetiole (Figs. 38 E, F) | Unnamed genus PH01 |
| 13(11) | Propodeum unarmed; anterior clypeal margin with two angles, or teeth, bearing hairs (Figs. 35 E, F) | Solenopsis |
| – | Propodeum with a pair of spines or teeth; anterior clypeal margin simple | 14 |
| 14(13) | Clypeus with 2 longitudinal carinae immediately anterior to antennal sockets; workers dimorphic (Figs. 13 C, D) | Carebara |
| – | Clypeus smooth and without longitudinal carinae; workers polymorphic (Figs. 29 E, F) | Pheidologeton |
| 15(9) | Antenna with 7–9 segments | 16 |
| – | Antenna with 10–12 segments | 17 |
| 16(15) | Antenna7-segmented; mesosoma strongly reticulate; propodeal spines long; anterior peduncle of petiole as long as petiolar node height (Figs. 24 E, F) | Myrmicaria |
| – | Antenna 9-segmented; mesosoma weakly reticulate and shield-like; propodeal spines short; anterior peduncle shorter than petiolar node height (Figs. 22 E, F) | Meranoplus |
| 17(15) | Eyes absent | 18 |
| – | Eyes present, even if only one ommatidium | 19 |
| 18(17) | Petiole and postpetiole with prominent anteroventral processes; integument firm and sclerotized (Figs. 21 C, D) | Liomyrmex |
| – | Petiole and postpetiole lacking anteroventral processes; integument fragile and poorly sclerotized (Figs. 10 C, D) | Anillomyrma |
| 19(17) | In dorsal view, postpetiole swollen and at least twice as wide as petiole; antenna 12-segmented (Figs. 13 A, B) | Cardiocondyla |
| – | In dorsal view, postpetiole as wide as, or slightly wider than, petiole; antenna 10–to 12-segmented | 20 |
| 20(19) | Clypeus with an anterior forked extension overhanging the mandibles; body covered with evenly-spaced clavate hairs (Figs. 12 C, D) | Calyptomyrmex |
| – | Clypeus without an anterior forked extension; body hairs not clavate | 21 |
| 21(20) | Sides of clypeus immediately anterior to antennal sockets produced into a sharp ridge | 22 |
| – | Sides of clypeus immediately anterior to antennal sockets not produced into a sharp ridge | 23 |
| 22(21) | In frontal view, head roughly heart-shaped, with posterior margin of the head strongly concave; antenna 12-segmented (Figs. 34 C, D) | Rhoptromyrmex |
| – | In frontal view, head roughly rectangular, with posterior margin of the head at most weakly concave; antenna 10–to 12-segmented (Figs. 37 A, B) | Tetramorium |
| 23(21) | Anterior clypeal margin with a single median seta extending over the mandibles; propodeum at most with blunt angles; (Figs. 23 C, D) | Monomorium |
| – | Anterior clypeal margin with a pair of setae, or a series of long, strong setae, or hairless; propodeum usually armed with spines | 24 |
| 24(23) | Antenna 11-segmented | 25 |
| – | Antenna 12-segmented | 30 |
| 25(24) | Antennal sockets completely exposed; anterior clypeal margin denticulate (Figs. 32 C, D) | Pristomyrmex |
| – | Antennal sockets partially or completely covered by frontal lobes; anterior clypeal margin usually simple | 26 |
| 26(25) | Antennal scrobes deep and narrow, overhung by expanded frontal carinae (Figs. 23 A, B) | Metapone |
| – | Antennal scrobes absent or very shallow | 27 |
| 27(26) | Propodeum unarmed; in profile, petiole lacking peduncle and usually with a large subpetiolar process (Figs. 38 A, B) | Vollenhovia (part) |
| – | Propodeum spinose; in profile, petiole pedunculate | 28 |
| 28(27) | In profile, propodeal spines curving upward and forward (Figs. 33 E, F) | Recurvidris |
| – | In profile, propodeal spines straight | 29 |
| 29(28) | Propodeal spines long and sharp; in side view, pronotum convex, much higher than propodeum (Figs. 21 E, F) | Lophomyrmex |
| – | Propodeal spines short and blunt; in side view, pronotum flat, almost level with propodeum (Figs. 18 A, B) | Gauromyrmex |
| 30(24) | Antenna gently expanding distally, with a weak to indistinct 4-segmented club; head strongly narrowed posteriorly (Figs. 11 E, F) | Aphaenogaster |
| – | Antenna abruptly expanding distally, with a distinct 3-segmented club; head not narrow posteriorly | 31 |
| 31(30) | Petiole lacking a peduncle | 32 |
| – | Petiole pedunculate | 34 |
| 32(31) | Propodeum armed with stout spines (curved in dorsal view) (Figs. 35 A, B) | Romblonella |
| – | Propodeum unarmed or at most with denticles | 33 |
| 33(32) | Petiole with a conspicuous ventral process; mandibles curved mesad and without a stem at insertion (Figs. 38 A, B) | Vollenhovia (part) |
| – | Petiole without a conspicuous ventral process; mandibles linear-triangular and with a stem at insertion (see diagnosis in “Brief Generic Accounts”) (Figs. 39 E, F) | Unnamed genus PH03 |
| 34(32) | Anterior clypeal margin overhanging mandibles and armed with blunt denticles or a median rectangular extension; in the major worker, head enormous, obscuring pronotum in dorsal view (Figs. 9 A, B) | Acanthomyrmex |
| – | Anterior clypeal margin not overhanging mandibles and simple; in the major worker, if present, head does not obscure pronotum in dorsal view | 35 |
| 35(34) | In profile, mesosomal dorsum with a flat or weakly convex outline; petiolar node blocky or roughly rectangular (Figs. 28 E, F) | Paratopula |
| – | In profile, dorsal mesosomal dorsum with a strongly convex outline; petiolar node globular or rounded | 36 |
| 36(35) | In profile, petiolar spiracle located on node, not on peduncle (see diagnosis in “Brief Generic Accounts”) (Figs. 39 A, B) | Unnamed genus PH02 |
| – | In profile, petiolar spiracle located on peduncle | 37 |
| 37(36) | Mandible with 5 teeth with a long diastema (toothless section) between 3rd and 4th tooth; side of head usually with a sinuate longitudinal groove running from the posterior margin to the mandibular insertion (Figs. 38 C, D) | Vombisidris |
| – | Mandibular dentition not as above; sinuate longitudinal groove at side of head absent | 38 |
| 38(37) | In profile, petiole with a short anterior peduncle; workers monomorphic (Figs. 22 A, B) | Lordomyrma |
| – | In profile, petiole with a long anterior peduncle; workers dimorphic (Figs. 29 C, D) | Pheidole |
| 1 | In side view, antennal scrobe present below eye | 2 |
| – | In side view, antennal scrobe absent or present above eye | 3 |
| 2(1) | Antenna 7-segmented; antennal scape triangular, widest near insertion and tapering distally; petiole pedunculate; propodeum armed with thin longitudinal flanges (Figs. 12 A, B) | Basiceros |
| – | Antenna 11-segmented; antennal scape rod-like; petiole sessile; propodeum armed with 2 thick spines (Figs. 13 E, F) | Cataulacus |
| 3(1) | Petiole lacking a distinct node | 4 |
| – | Petiole with a distinct node | 6 |
| 4(3) | Petiole transversely flattened; in dorsal view, in dorsal view, gaster roughly heart-shaped; (Figs. 15 A, B) | Crematogaster |
| – | Petiole roughly barrel-shaped; in dorsal view, gaster not heart-shaped | 5 |
| 5(4) | Side of head with a longitudinal ridge running below the eye, from rear border to mandibular insertion; posterolateral corner of the head not forming a blunt point (Figs. 24 C, D) | Myrmecina |
| – | Side of head lacking a ridge below the eye; posterolateral corner of the head forming a blunt point (Figs. 16 A, B) | Dilobocondyla |
| 6(3) | Mandibles bidentate (with 2 teeth); mesosoma without a metanotal groove (Figs. 37 E, F) | Tyrannomyrmex |
| – | Mandibles with more than 2 teeth; mesosoma usually with a metanotal groove | 7 |
| 7(6) | Petiole and postpetiole with at least some light-colored sponge-like tissue | 8 |
| – | Petiole and postpetiole never with sponge-like tissue | 9 |
| 8(7) | Sponge-like tissue only on ventral surface of petiole, postpetiole, and the 4th abdominal segment; antenna 11-segmented (Figs. 15 C, D) | Dacetinops |
| – | Sponge-like tissue on lateral, as well as ventral, surfaces of petiole, postpetiole, and 4th abdominal segment; antenna 4–to 6-segmented (Figs. 36 A, B) | Strumigenys |
| 9(7) | Antenna with a distal club of 2 segments | 10 |
| – | Antenna with a distal club of 3 or 4 segments, or with an indistinct club | 15 |
| 10(9) | Antennal scrobe present; head and mesosoma punctuate (Figs. 22 C, D) | Mayriella |
| – | Antennal scrobe absent; head and mesosoma smooth, rugose, or reticulate | 11 |
| 11(10) | Head and mesosoma sculptured; petiole at most with a short peduncle | 12 |
| – | Head and mesosoma smooth between sparse punctures; petiole pedunculate | 13 |
| 12(11) | Head and mesosoma longitudinally rugose; propodeum unarmed; gaster broadly attached to postpetiole (Figs. 34 A, B) | Rhopalomastix |
| – | Head and mesosoma strongly reticulate; propodeum bidentate; gaster narrowly attached to postpetiole (Figs. 38 E, F) | Unnamed genus PH01 |
| 13(11) | Propodeum unarmed; anterior clypeal margin with two angles, or teeth, bearing hairs (Figs. 35 E, F) | Solenopsis |
| – | Propodeum with a pair of spines or teeth; anterior clypeal margin simple | 14 |
| 14(13) | Clypeus with 2 longitudinal carinae immediately anterior to antennal sockets; workers dimorphic (Figs. 13 C, D) | Carebara |
| – | Clypeus smooth and without longitudinal carinae; workers polymorphic (Figs. 29 E, F) | Pheidologeton |
| 15(9) | Antenna with 7–9 segments | 16 |
| – | Antenna with 10–12 segments | 17 |
| 16(15) | Antenna7-segmented; mesosoma strongly reticulate; propodeal spines long; anterior peduncle of petiole as long as petiolar node height (Figs. 24 E, F) | Myrmicaria |
| – | Antenna 9-segmented; mesosoma weakly reticulate and shield-like; propodeal spines short; anterior peduncle shorter than petiolar node height (Figs. 22 E, F) | Meranoplus |
| 17(15) | Eyes absent | 18 |
| – | Eyes present, even if only one ommatidium | 19 |
| 18(17) | Petiole and postpetiole with prominent anteroventral processes; integument firm and sclerotized (Figs. 21 C, D) | Liomyrmex |
| – | Petiole and postpetiole lacking anteroventral processes; integument fragile and poorly sclerotized (Figs. 10 C, D) | Anillomyrma |
| 19(17) | In dorsal view, postpetiole swollen and at least twice as wide as petiole; antenna 12-segmented (Figs. 13 A, B) | Cardiocondyla |
| – | In dorsal view, postpetiole as wide as, or slightly wider than, petiole; antenna 10–to 12-segmented | 20 |
| 20(19) | Clypeus with an anterior forked extension overhanging the mandibles; body covered with evenly-spaced clavate hairs (Figs. 12 C, D) | Calyptomyrmex |
| – | Clypeus without an anterior forked extension; body hairs not clavate | 21 |
| 21(20) | Sides of clypeus immediately anterior to antennal sockets produced into a sharp ridge | 22 |
| – | Sides of clypeus immediately anterior to antennal sockets not produced into a sharp ridge | 23 |
| 22(21) | In frontal view, head roughly heart-shaped, with posterior margin of the head strongly concave; antenna 12-segmented (Figs. 34 C, D) | Rhoptromyrmex |
| – | In frontal view, head roughly rectangular, with posterior margin of the head at most weakly concave; antenna 10–to 12-segmented (Figs. 37 A, B) | Tetramorium |
| 23(21) | Anterior clypeal margin with a single median seta extending over the mandibles; propodeum at most with blunt angles; (Figs. 23 C, D) | Monomorium |
| – | Anterior clypeal margin with a pair of setae, or a series of long, strong setae, or hairless; propodeum usually armed with spines | 24 |
| 24(23) | Antenna 11-segmented | 25 |
| – | Antenna 12-segmented | 30 |
| 25(24) | Antennal sockets completely exposed; anterior clypeal margin denticulate (Figs. 32 C, D) | Pristomyrmex |
| – | Antennal sockets partially or completely covered by frontal lobes; anterior clypeal margin usually simple | 26 |
| 26(25) | Antennal scrobes deep and narrow, overhung by expanded frontal carinae (Figs. 23 A, B) | Metapone |
| – | Antennal scrobes absent or very shallow | 27 |
| 27(26) | Propodeum unarmed; in profile, petiole lacking peduncle and usually with a large subpetiolar process (Figs. 38 A, B) | Vollenhovia (part) |
| – | Propodeum spinose; in profile, petiole pedunculate | 28 |
| 28(27) | In profile, propodeal spines curving upward and forward (Figs. 33 E, F) | Recurvidris |
| – | In profile, propodeal spines straight | 29 |
| 29(28) | Propodeal spines long and sharp; in side view, pronotum convex, much higher than propodeum (Figs. 21 E, F) | Lophomyrmex |
| – | Propodeal spines short and blunt; in side view, pronotum flat, almost level with propodeum (Figs. 18 A, B) | Gauromyrmex |
| 30(24) | Antenna gently expanding distally, with a weak to indistinct 4-segmented club; head strongly narrowed posteriorly (Figs. 11 E, F) | Aphaenogaster |
| – | Antenna abruptly expanding distally, with a distinct 3-segmented club; head not narrow posteriorly | 31 |
| 31(30) | Petiole lacking a peduncle | 32 |
| – | Petiole pedunculate | 34 |
| 32(31) | Propodeum armed with stout spines (curved in dorsal view) (Figs. 35 A, B) | Romblonella |
| – | Propodeum unarmed or at most with denticles | 33 |
| 33(32) | Petiole with a conspicuous ventral process; mandibles curved mesad and without a stem at insertion (Figs. 38 A, B) | Vollenhovia (part) |
| – | Petiole without a conspicuous ventral process; mandibles linear-triangular and with a stem at insertion (see diagnosis in “Brief Generic Accounts”) (Figs. 39 E, F) | Unnamed genus PH03 |
| 34(32) | Anterior clypeal margin overhanging mandibles and armed with blunt denticles or a median rectangular extension; in the major worker, head enormous, obscuring pronotum in dorsal view (Figs. 9 A, B) | Acanthomyrmex |
| – | Anterior clypeal margin not overhanging mandibles and simple; in the major worker, if present, head does not obscure pronotum in dorsal view | 35 |
| 35(34) | In profile, mesosomal dorsum with a flat or weakly convex outline; petiolar node blocky or roughly rectangular (Figs. 28 E, F) | Paratopula |
| – | In profile, dorsal mesosomal dorsum with a strongly convex outline; petiolar node globular or rounded | 36 |
| 36(35) | In profile, petiolar spiracle located on node, not on peduncle (see diagnosis in “Brief Generic Accounts”) (Figs. 39 A, B) | Unnamed genus PH02 |
| – | In profile, petiolar spiracle located on peduncle | 37 |
| 37(36) | Mandible with 5 teeth with a long diastema (toothless section) between 3rd and 4th tooth; side of head usually with a sinuate longitudinal groove running from the posterior margin to the mandibular insertion (Figs. 38 C, D) | Vombisidris |
| – | Mandibular dentition not as above; sinuate longitudinal groove at side of head absent | 38 |
| 38(37) | In profile, petiole with a short anterior peduncle; workers monomorphic (Figs. 22 A, B) | Lordomyrma |
| – | In profile, petiole with a long anterior peduncle; workers dimorphic (Figs. 29 C, D) | Pheidole |
| 1 | Mandibles long and linear, inserted at middle of the anterior margin of head | 2 |
| – | Mandibles triangular or linear, inserted at sides of head | 3 |
| 2(1) | Frons with a conspicuous groove running along the midline; in profile, petiole node produced into a dorsal sharp point (Figs. 26 E, F) | Odontomachus |
| – | Frons simple, at most with shallow striations; in profile, petiole node blunt (Figs. 10 E, F) | Anochetus |
| 3(1) | Antennal insertions well separated; hind tibia with two pectinate spurs (Figs. 30 E, F) | Platythyrea |
| – | Antennal insertions closely approximated; hind tibia with one pectinate spur, but may have a smaller simple spur in front of it | 4 |
| 4(3) | Hind tibia with only one pectinate spur, without a smaller, simple anterior spur | 5 |
| – | Hind tibia with two spurs with the smaller spur simple | 8 |
| 5(4) | Side of mandible near insertion with a small circular pit (Figs. 28 A, B) | Pachycondyla (part) |
| – | Side of mandible near insertion without a pit | 6 |
| 6(5) | Outer surface of middle tibia and middle and hind tarsi with strong, peg-like teeth; pronotum flat and shelf-like in profile (Figs. 14 A, B) | Centromyrmex |
| – | Outer surface of middle tibia and middle and hind tarsi with hairs, but never with strong, peg-like teeth; pronotum convex in profile | 7 |
| 7(6) | Subpetiolar process with an oval or circular translucent window, and with a sharp posterior angle (Figs. 31 C, D) | Ponera |
| – | Subpetiolar process without an oval or circular translucent window, and usually blunt or rounded posteriorly (Figs. 19 C, D) | Hypoponera |
| 8(4) | Tarsal claws on hind leg either pectinate or with one or more teeth on inner surface | 9 |
| – | Tarsal claws on hind leg simple, never with teeth on inner surface | 10 |
| 9(8) | Ocelli present; mandibles long and forceps-like with a triangular flange beneath (Figs. 19 A, B) | Harpegnathos |
| – | Ocelli absent; mandibles variable but never long and forceps-like with a triangular flange beneath (Figs. 20 D, E) | Leptogenys |
| 10(8) | Petiole node with a pair of spines (Figs. 15 C, D) | Diacamma |
| – | Petiole node simple | 11 |
| 11(10) | Side of pronotum with a pair of large blunt angles; anterior clypeal margin with small blunt teeth or denticles (Figs. 27 A, B) | Odontoponera |
| – | Side of pronotum and anterior clypeal margin simple | 12 |
| 12(11) | Mandibles, when fully closed, with a large gap between them; eyes situated very near base of mandibles (Figs. 23 E, F) | Myopias |
| – | Mandibles, when fully closed, slightly overlap along the inner margin; eyes situated away from base of mandibles (Figs. 28 A, B) | Pachycondyla (part) |
| 1 | Mandibles long and linear, inserted at middle of the anterior margin of head | 2 |
| – | Mandibles triangular or linear, inserted at sides of head | 3 |
| 2(1) | Frons with a conspicuous groove running along the midline; in profile, petiole node produced into a dorsal sharp point (Figs. 26 E, F) | Odontomachus |
| – | Frons simple, at most with shallow striations; in profile, petiole node blunt (Figs. 10 E, F) | Anochetus |
| 3(1) | Antennal insertions well separated; hind tibia with two pectinate spurs (Figs. 30 E, F) | Platythyrea |
| – | Antennal insertions closely approximated; hind tibia with one pectinate spur, but may have a smaller simple spur in front of it | 4 |
| 4(3) | Hind tibia with only one pectinate spur, without a smaller, simple anterior spur | 5 |
| – | Hind tibia with two spurs with the smaller spur simple | 8 |
| 5(4) | Side of mandible near insertion with a small circular pit (Figs. 28 A, B) | Pachycondyla (part) |
| – | Side of mandible near insertion without a pit | 6 |
| 6(5) | Outer surface of middle tibia and middle and hind tarsi with strong, peg-like teeth; pronotum flat and shelf-like in profile (Figs. 14 A, B) | Centromyrmex |
| – | Outer surface of middle tibia and middle and hind tarsi with hairs, but never with strong, peg-like teeth; pronotum convex in profile | 7 |
| 7(6) | Subpetiolar process with an oval or circular translucent window, and with a sharp posterior angle (Figs. 31 C, D) | Ponera |
| – | Subpetiolar process without an oval or circular translucent window, and usually blunt or rounded posteriorly (Figs. 19 C, D) | Hypoponera |
| 8(4) | Tarsal claws on hind leg either pectinate or with one or more teeth on inner surface | 9 |
| – | Tarsal claws on hind leg simple, never with teeth on inner surface | 10 |
| 9(8) | Ocelli present; mandibles long and forceps-like with a triangular flange beneath (Figs. 19 A, B) | Harpegnathos |
| – | Ocelli absent; mandibles variable but never long and forceps-like with a triangular flange beneath (Figs. 20 D, E) | Leptogenys |
| 10(8) | Petiole node with a pair of spines (Figs. 15 C, D) | Diacamma |
| – | Petiole node simple | 11 |
| 11(10) | Side of pronotum with a pair of large blunt angles; anterior clypeal margin with small blunt teeth or denticles (Figs. 27 A, B) | Odontoponera |
| – | Side of pronotum and anterior clypeal margin simple | 12 |
| 12(11) | Mandibles, when fully closed, with a large gap between them; eyes situated very near base of mandibles (Figs. 23 E, F) | Myopias |
| – | Mandibles, when fully closed, slightly overlap along the inner margin; eyes situated away from base of mandibles (Figs. 28 A, B) | Pachycondyla (part) |
| 1 | Fourth abdominal segment straight or slightly curved, so that apex of gaster is directed posteriorly (Figs. 32 E, F) | Probolomyrmex |
| – | Fourth abdominal segment strongly curved, so that apex of gaster is directed anteriorly | 2 |
| 2(1) | Apical segment of antenna extremely large and bulbous; antennal sockets on shelf protruding over mandibles (Figs. 16 C, D) | Discothyrea |
| – | Apical segment moderately enlarged, but not bulbous; antennal sockets not protruding over mandibles (Figs. 33 A, B) | Proceratium |
| 1 | Fourth abdominal segment straight or slightly curved, so that apex of gaster is directed posteriorly (Figs. 32 E, F) | Probolomyrmex |
| – | Fourth abdominal segment strongly curved, so that apex of gaster is directed anteriorly | 2 |
| 2(1) | Apical segment of antenna extremely large and bulbous; antennal sockets on shelf protruding over mandibles (Figs. 16 C, D) | Discothyrea |
| – | Apical segment moderately enlarged, but not bulbous; antennal sockets not protruding over mandibles (Figs. 33 A, B) | Proceratium |
Abdominal segments – morphologically, segment I of the ant abdomen is the propodeum (see below), followed by the petiole (see below), which is segment II. The postpetiole (see below), when present, is segment III. Otherwise, the first segment of the gaster (see below) is segment III.
Acidopore – at the apex of the gaster (see below), a round, somewhat raised, orifice, often fringed with hairs at the tip; diagnostic of ants belonging to subfamily Formicinae.
Angle – a triangular, broad-based, tooth-like extension of the cuticle.
Antennal club – describes an antennal funiculus that enlarges apically, to form a distinct club of 2-4 segments.
Antennal funiculus – the portion of the antenna distal to the antennal scape; composed of 3-12 segments of varying size.
Antennal scape – the elongated basal segment of the antenna.
Antennal scrobe – a groove above or below the eye, which accepts the folded antenna.
Anterior clypeal margin – the leading edge of the clypeus (see below).
Bidentate – armed with 2 teeth (mandibles) or short spines (propodeum).
Clavate – refers to hairs that are blunt and club-shaped.
Clypeus – the anterior sclerite (see below) of the dorsal head, which consists of narrow lateral portions and a shield-like median portion.
Coxa – see Leg segments.
Declivity – downward slope, eg. as of the propodeum.
Dentate – describes mandibles armed with teeth.
Denticle – a small triangular tooth, much reduced in size.
Denticulate – armed with denticles.
Edentate – describes mandibles without teeth or denticles on the inner margin.
Epigaeic – describes ants that live above ground (compare with hypogaeic).
Filiform – describes an antennal funiculus with segments of approximately the same size (compare with antennal club).
Frons – the region of the head extending from behind the clypeus, and between the eyes to the posterior margin of the head.
Frontal carina – (pl. carinae) thin ridges of cuticle on the front of the head, which may form the dorsal margins of the antennal scrobes (see above).
Frontal lobe – projection of the frontal carina (see above), which may partially or completely cover the antennal sockets.
Gaster – old name for the third main body division of the ant body.
Head – the first main body division of the ant body.
Hypogaeic – describes ants that live underground (compare with epigaeic).
Labial palps – the segmented sensory appendages attached to the labium, found on the anteroventral surface of the head, with a maximum of four segments. These are the inner pair of palps.
Leg segments – Each leg consists of the basal coxa, a small trochanter, a usually long and swollen femur, a tibia, anda 5-segmented tarsus, ending in a pair of tarsal claws. Pro-, meso-, and meta- are prefixes that indicate the leg segment of the particular thoracic segment.
Mandibles – anterior appendages of the head, with which the ant manipulates its environment. They are variable in shape, dentition, and function, and extremely important in ant taxonomy.
Maxillary palps – the segmented sensory appendages attached to the maxillae, found on the anteroventral surface of the head. With a maximum of six segments, they are usually the longer and larger of the palps and are the outer pair of palps.
Mesosoma – (= alitrunk) the second main body division of the ant body. Morphologically, it is composed of the three thoracic segments (pro-, meso-, and metathorax) to which is fused the propodeum (see below).
Mesonotum – the second tergite (see below) of the mesosoma (see above).
Mesotibia – see Leg segments.
Metanotal groove – a transverse depression between the mesonotum (see above) and the propodeum (see below).
Metapleural gland – an exocrine gland found on the posteroventral side of the mesosoma (see above), just above the metacoxa (see Leg segments). This is one of the defining features of ants, although it has been secondarily lost or reduced in some taxa.
Node – a prominent bulge on the dorsal surface of the petiole.
Ocellus – (pl. ocelli) a simple eye found on the frons (see above) of the head, usually in reproductives but may also be found in the workers of some taxa.
Ommatidium – an individual facet of the compound eye.
Palp formula – a standardized method of giving the number of segments of the maxillary and labial palps. The number of maxillary palp (see above) segments is given first, followed by the number of labial palp (see above) segments; thus “PF 6, 4” indicates that there are six maxillary palp segments and four labial palp segments.
Pectinate – describes tarsal claws or tarsal spurs that are comb-like.
Pedunculate – bearing a stem-like projection, or peduncle, that is anterior to the petiolar node (compare with sessile and sub-sessile).
Petiole – the isolated segment separating the mesosoma (see above) and the gaster (see above). This is one of the defining features of ants. Morphologically, it is the second segment of the abdomen.
Postpetiole – the second isolated and reduced segment separating the mesosoma (see above) and the gaster (see above), in ants with a 2-segmented waist. Morphologically, it is the third abdominal segment.
Pronotum – the first tergite (see below) of the mesosoma (see above).
Propodeum – (= epinotum) the dorsal posterior plate of the mesosoma (see above). Morphologically, it is the first segment of the abdomen, fused to the thorax. It may have specializations such as spines, teeth, or lobes.
Punctate – describes surface sculpturing composed of round pits which may be shallow or deep.
Reticulate – describes surface sculpturing composed of an irregular network of thin ridges of cuticle.
Rugose – describes surface sculpturing of raised ridges without cross-ridges.
Sclerite – a hardened or stiffened plate of the integument.
Sculpturing – features on the body surface which may be pits, grooves, striae, ridges, punctures, or a combination.
Sessile – describes a petiole without a peduncle, such that the petiolar node is very close to the propodeum (compare with pedunculate).
Shagreened – describes body surface that has a dull, light-absorbing quality.
Spinose – armed with spines.
Sternite – the lower or ventral sclerite (see above) of a segment.
Striate – describes sculpturing composed of shallow, parallel grooves or lines.
Tergite – the upper or dorsal sclerite (see above) of a segment.
We would like to thank Fr. Joel E. Tabora, S.J., former president of Ateneo de Naga University, for loaning the ant specimens to the Museum of Comparative Zoology on long-term basis, and Emelina G. Regis (INECAR), for allowing us the use of her office space and laboratory equipment during our transect work. We also thank Joanaviva Caceres-Plopenio (Ateneo de Naga University), Perry Buenavente (Ateneo de Manila University), Arvin Diesmos and Ven Samarita (Philippine National Museum, Manila), Ireneo Lit, Jr. and Leonila A.C. Raros (UPLB-Museum of Natural History), Herbert Zettel (Museum of Natural History, Vienna), Ted Schultz (Smithsonian Institution), Hendrik Freitag, and Keith Arakaki (Bernice P. Bishop Museum) for giving us access to their specimens. For permitting us to use their images, we thank Katsuyuki Eguchi for Anillomyrma decamera, first published in Myrmecological News (Eguchi et al. 2010), Hans Peter Ravn for Noonilla copiosa, first published in Entomologiske Meddelelser (Petersen 1968), Martin Pfeiffer, NHMW and www.antbase.net for Vombisidris philippina, first published in Entomologica Austriaca (Zettel and Sorger 2010a), and Arvin Diesmos and Perry Buenavente for habitat images.We are also very grateful to Denise R. General for post-processing the images and preparing the image plates. We also thank Stefan Cover and the late Roy Snelling for reviewing our paper. Finally, we thank Brian Fisher, Steve Shattuck and an anonymous reviewer for their very helpful suggestions in greatly improving our paper.
There are 474 ant species and subspecies known from the Philippines and at least an additional 100 undescribed or unidentified species represented by museum specimens and recent collections. The total size of the ant fauna is no doubt much larger, almost certainly more than 1000 species are present. This tentative list contains species and subspecies names regarded as valid in Bolton (2011) and later publications. It is meant to serve as a rough guide to our current knowledge of the Philippine ant fauna. Keys to the notes and island codes are at the end of the list.
| Species | Distribution | Notes |
|---|---|---|
| Acanthomyrmex mindanao Moffett, 1986 | Md | |
| Acropyga acutiventris Roger, 1862 | Md | |
| Acropyga nipponensis Terayama, 1985 | Pl | |
| Acropyga pallida (Donisthorpe, 1938) | Lz | A/G – new record |
| Aenictus alticola Wheeler & Chapman (in Wheeler, 1930c) | Lz | |
| Aenictus bakeri Menozzi, 1925 | Md | |
| Aenictus camposi Wheeler & Chapman, 1925 | Lz, Ng | |
| Aenictus carolianus Zettel & Sorger, 2010b | Cb | |
| Aenictus ceylonicus (Mayr, 1866a) | Lz, Ng | |
| Aenictus gracilis Emery, 1893b | Lz, Md, Mr, Ng | |
| Aenictus laeviceps (F. Smith, 1857) | Ly, Lz, Md, Ng | |
| Aenictus luzoni Wheeler & Chapman, 1925 | Lz, Ng | |
| Aenictus nesiotis Wheeler & Chapman (in Wheeler, 1930c) | Lz, Ng, Pl | |
| Aenictus pangantihoni Zettel & Sorger, 2010b | Cm | |
| Aenictus philippinensis Chapman, 1963 | Ng | |
| Aenictus piercei Wheeler & Chapman (in Wheeler, 1930c) | Md, Ng | |
| Aenictus powersi Wheeler & Chapman (in Wheeler, 1930c) | Ng | |
| Aenictus rabori Chapman, 1963 | Ng | |
| Aenictus reyesi Chapman, 1963 | Ng | |
| Anillomyrma decamera (Emery, 1901b) | Lz | |
| Anochetus brevis Brown, 1978 | Md | |
| Anochetus cato Forel, 1901 | ||
| Anochetus graeffei Mayr, 1870 | Lz, Ms, Ng, Pl | |
| Anochetus incultus Brown, 1978 | Lz | |
| Anochetus isolatus Mann, 1919 | Lz, Ng | |
| Anochetus leyticus Zettel, 2012 | Ly | |
| Anochetus modicus Brown, 1978 | Lz, Ng | |
| Anochetus pangantihoni Zettel, 2012 | Ly | |
| Anochetus princeps Emery, 1884 | Lz | |
| Anochetus ruginotis Stitz, 1925 | Lz, Md | |
| Anochetus schoedli Zettel, 2012 | Lz | |
| Anochetus turneri Forel, 1900b | Lz | |
| Anochetus werneri Zettel, 2012 | Md | |
| Anomalomyrma helenae Borowiec, Schulz, Alpert & Baňař, 2011 | Pl | |
| Anoplolepis gracilipes (F. Smith, 1857) | W | |
| Basiceros chapmani (Taylor, 1990b) | Lz | |
| Basiceros philippinum (Brown & Kempf, 1960) | Lz, Ng | |
| Basiceros procerum (Emery, 1897a) | W | |
| Calyptomyrmex beccarii Emery, 1887b | Lz, Ng | |
| Calyptomyrmex loweryi Shattuck, 2011 | Lz, Md | |
| Camponotus albocinctus (Ashmead, 1905) | Lz | |
| Camponotus barbatus Roger, 1863 | ||
| Camponotus barbosus Baroni Urbani, 1971 | Lz, Md | |
| Camponotus bellus leucodiscus Wheeler, 1919a | Lz | |
| Camponotus carin Emery, 1889 | Lz | |
| Camponotus castanicola Donisthorpe, 1943b | Lz, Md | |
| Camponotus cinerascens (Fabricius, 1787) | ||
| Camponotus compressus (Fabricius, 1787) | Pl | |
| Camponotus corallinus (Roger, 1863) | Lz, Md | |
| Camponotus gigas (Latreille, 1802) | Lz | |
| Camponotus horrens Forel, 1910 | Lz | |
| Camponotus irritans pallidus (F. Smith, 1857) | Lz | |
| Camponotus japonicus Mayr, 1866b | Md | |
| Camponotus karawaiewi Menozzi, 1926 | Md | |
| Camponotus leonardi Emery, 1889 | Lz, Ng | |
| Camponotus leonardi gracilentus Stitz, 1925 | Lz | |
| Camponotus maculatus foveolatus Stitz, 1925 | ||
| Camponotus maculatus subnudus Stitz, 1925 | Lz, Pl | |
| Camponotus nicobarensis monticola Bingham, 1903 | Lz | |
| Camponotus nigricans Roger, 1863 | Jo, Lz | |
| Camponotus platypus Roger, 1863 | Lz, Md | |
| Camponotus pressipes Emery, 1893c | Md | |
| Camponotus quadrisectus (F. Smith, 1858) | Md, Pl | |
| Camponotus reticulatus jagori Stitz, 1925 | Lz | |
| Camponotus rothneyi makilingi Viehmeyer, 1916b | Lz | |
| Camponotus rufifemur obscurus Stitz, 1925 | Lz | |
| Camponotus solenobius Menozzi, 1926 | Md | |
| Camponotus trietericus Menozzi, 1926 | Lz | |
| Camponotus variegatus crassinodis Forel, 1892a | Ng | |
| Camponotus vitreus (F. Smith, 1860b) | W | |
| Cardiocondyla kagutsuchi Terayama, 1999 | By, Lz | |
| Cardiocondyla mauritanica (Forel, 1890) | Lz | |
| Cardiocondyla sima Wheeler, 1935b | Lz, Md | |
| Cardiocondyla tjibodana Karavaiev, 1935 | By, Lz | |
| Cardiocondyla wroughtonii (Forel, 1890) | Lz | |
| Carebara alperti Fernandez, 2010 | Ng | |
| Cataulacus catuvolcus Bolton, 1974 | Lz, Rm | |
| Cataulacus chapmani Bolton, 1974 | Lz, Ng | |
| Cataulacus setosus F. Smith, 1860b | Md | |
| Centromyrmex feae (Emery, 1889) | Bs, Lz | |
| Cerapachys bakeri (Wheeler & Chapman, 1925) | Bs | |
| Cerapachys biroi Forel, 1907 | ||
| Cerapachys crawleyi Wheeler, 1924b | Lz, Ng | |
| Cerapachys jacobsoni Forel, 1912a | Ng | |
| Cerapachys longitarsus (Mayr, 1879) | Lz | |
| Cerapachys luzuriagae (Wheeler & Chapman, 1925) | Ng | |
| Cerapachys muiri Wheeler & Chapman, 1925 | Lz, Md | |
| Cerapachys pruinosus Brown, 1975 | Ng | |
| Cerapachys rufithorax Wheeler & Chapman, 1925 | Lz, Ng | |
| Cerapachys sulcinodis Emery, 1889 | Lz, Ng | |
| Cerapachys suscitatus (Viehmeyer, 1913) | Ng | |
| Crematogaster ampullaris F. Smith, 1861 | Ly, Lz | |
| Crematogaster bakeri Menozzi, 1925 | Md | |
| Crematogaster bicolor imbellis Emery, 1893c | Lz, Ng | |
| Crematogaster brunnea ruginota Santschi, 1928a | ||
| Crematogaster crassicornis Emery, 1893c | Lz, Md | |
| Crematogaster cylindriceps Wheeler, 1927 | ||
| Crematogaster difformis F. Smith, 1857 | Lz | |
| Crematogaster inflata F. Smith, 1857 | Lz | |
| Crematogaster longiclava Emery, 1893c | Lz | |
| Crematogaster modiglianii Emery, 1900b | Md, Ng | |
| Crematogaster ochracea Mayr, 1862 | Lz, Ng | |
| Crematogaster onusta Stitz, 1925 | Pl, SC | |
| Crematogaster rogenhoferi Mayr, 1879 | Lz | |
| Crematogaster semperi Emery, 1893c | Lz | |
| Crematogaster simoni Emery, 1893c | Lz | |
| Crematogaster vitalisi Menozzi, 1925 | Md | |
| Dacetinops cirrosus Taylor, 1985 | Ly | HZW – new record |
| Diacamma baguiense Wheeler & Chapman, 1925 | Lz | |
| Diacamma palawanicum concentricum Wheeler & Chapman, 1925 | Pl | |
| Diacamma palawanicum Emery, 1900b | Pl | |
| Diacamma panayense Wheeler & Chapman, 1925 | Pn | |
| Diacamma rugosum (Le Guillou, 1842) | W | |
| Diacamma rugosum viridipurpureum Emery, 1893c | Lz | |
| Diacamma sericeiventre Stitz, 1925 | Pl | |
| Dilobocondyla chapmani rufobrunnea Wheeler, 1935b | Ng | |
| Dilobocondyla chapmani Wheeler, 1924a | Ng | |
| Discothyrea bryanti (Wheeler, 1917) | Lz | |
| Discothyrea clavicornis Emery, 1897a | Lz | |
| Dolichoderus affinis Emery, 1889 | ||
| Dolichoderus patens pubiventris Emery, 1900b | Pl | |
| Dolichoderus thoracicus (F. Smith, 1860a) | W | |
| Echinopla pallipes F. Smith, 1857 | Lz | |
| Echinopla pseudostriata Donisthorpe, 1943a | Lz | |
| Echinopla striata aciculata (F. Smith, 1858) | ||
| Echinopla striata F. Smith, 1857 | ||
| Echinopla vermiculata Emery, 1898 | Lz | |
| Euprenolepis negrosensis (Wheeler, 1930b) | Ng | |
| Forelophilus philippinensis Zettel & Zimmerman, 2007 | Lz, Md | |
| Forelophilus stefanschoedli Zettel & Zimmerman, 2007 | Ly, Lz, Md | |
| Gauromyrmex acanthinus (Karavaiev, 1935) | MCZ – new record | |
| Gesomyrmex luzonensis (Wheeler, 1916) | Lz, Ng | |
| Gnamptogenys binghamii (Forel, 1900d) | Lz, Ng | |
| Gnamptogenys bulbopila Lattke, 2004 | Md | |
| Gnamptogenys chapmani Brown, 1958 | Lz, Ng | |
| Gnamptogenys costata (Emery, 1889) | Lz, Md | |
| Gnamptogenys cribrata (Emery, 1900a) | Lz, Ng | |
| Gnamptogenys fistulosa Lattke, 2004 | Lz | |
| Gnamptogenys laevior (Forel, 1905) | Lz | |
| Gnamptogenys leiolabia Lattke, 2004 | Ng | |
| Gnamptogenys luzonensis (Wheeler, 1929d) | Lz, Ng | |
| Gnamptogenys menadensis (Mayr, 1887) | Lz | |
| Gnamptogenys posteropsis (Gregg, 1951) | Lz, Md, Ng | |
| Harpegnathos empesoi Chapman, 1963 | Md | |
| Harpegnathos macgregori Wheeler & Chapman, 1925 | Bi | |
| Harpegnathos medioniger Donisthorpe, 1942 | Lz | |
| Harpegnathos venator chapmani Donisthorpe, 1937 | Lz | |
| Hypoponera confinis javana (Forel, 1905) | Lz, Ng | |
| Hypoponera opaciceps (Mayr, 1887) | ||
| Hypoponera pruinosa (Emery, 1900a) | Lz | |
| Hypoponera punctatissima (Roger, 1859) | Lz | |
| Hypoponera ragusai (Emery, 1894) | Lz | |
| Hypoponera sabronae (Donisthorpe, 1941b) | ||
| Iridomyrmex anceps (Roger, 1863) | Mq | |
| Iridomyrmex angusticeps Forel, 1901 | Md | |
| Lepisiota aurea punctaticeps Wheeler, 1935b | Lz, Ng | |
| Lepisiota chapmani Wheeler, 1935b | Ng | |
| Leptanilla astylina Petersen, 1968 | Pl | |
| Leptogenys chinensis (Mayr, 1870) | Ng | |
| Leptogenys diminuta (F. Smith, 1857) | Lz, Md, Ng, Pl | |
| Leptogenys falcigera Roger, 1861 | Ng | |
| Leptogenys iridescens (F. Smith, 1857) | Pl | |
| Leptogenys maxillosa (F. Smith, 1858) | Pn | |
| Leptogenys mutabilis (F. Smith, 1861) | Pl | |
| Leptogenys peuqueti (Andre, 1887) | Lz, Ng | |
| Leptogenys pruinosa Forel, 1900d | Lz, Ng | |
| Leptogenys punctiventris (Mayr, 1879) | Lz, Ng | |
| Leptogenys varicosa (Stitz, 1925) | Pl | |
| Leptogenys watsoni Forel, 1900d | Lz, Ng, Pn | |
| Leptomyrmex fragilis (F. Smith, 1859) | Md | BPBM – new record |
| Liomyrmex gestroi (Emery, 1887b) | W | |
| Lophomyrmex bedoti Emery, 1893b | Pl | |
| Lordomyrma diwata Taylor, 2012 | Lz | |
| Lordomyrma emarginata Taylor, 2012 | Lz | |
| Lordomyrma idianale Taylor, 2012 | Lz | |
| Lordomyrma limatula Taylor, 2012 | Ly | |
| Mayriella transfuga Baroni Urbani, 1977a | Lz | A/G – new record |
| Meranoplus biliran Schödl, 1998 | Bi | |
| Metapone bakeri Wheeler, 1916 | Lz | |
| Metapone gracilis Wheeler, 1935b | Md | |
| Monomorium banksi Forel, 1910 | Ng | |
| Monomorium destructor (Jerdon, 1851) | Lz | |
| Monomorium floricola (Jerdon, 1851) | Cm, Lz, Md, Tb | |
| Monomorium orientale Mayr, 1879 | ||
| Monomorium pharaonis (Linnaeus, 1758) | W | |
| Myopias bidens polita (Stitz, 1925) | Ng, Pl | |
| Myopias breviloba (Wheeler, 1919a) | Bs | |
| Myopias lobosa Willey & Brown, 1983 | Lz | |
| Myopias modiglianii (Emery 1900b) | Ng | |
| Myopias philippinensis (Menozzi, 1925) | Lz, Md | |
| Myopopone castanea (F. Smith 1860b) | Ly, Lz, Md, Pl | |
| Myrmicaria aphidicola Calilung, 2000 | Md | |
| Myrmicaria brunnea subcarinata (F. Smith, 1857) | W | |
| Myrmoteras glabrum Zettel & Sorger, 2011 | Cm | |
| Myrmoteras insulcatum Moffett, 1985 | Lz, Ng | |
| Myrmoteras mcarthuri Zettel & Sorger, 2011 | Ly | |
| Myrmoteras williamsi Wheeler, 1919a | Lz, Ng | |
| Mystrium camillae Emery, 1889 | Lz, Pl | |
| Noonilla copiosa Petersen, 1968 | Pl | |
| Ochetellus glaber (Mayr, 1862) | Lz, Ng | MCZ – new record |
| Odontomachus alius Sorger & Zettel, 2011 | Bh, Bi, Cb, Ct, Ly, Sm | |
| Odontomachus banksi Forel, 1910 | Cb, Lz | |
| Odontomachus infandus F. Smith, 1858 | Lz, Mr, Ng, Rm | |
| Odontomachus malignus F. Smith, 1859 | Ba, Bh, Jo, Tw | |
| Odontomachus papuanus Emery, 1887b | Ng, Pl, Pn | |
| Odontomachus philippinus Emery, 1893b | Ng, Pn, Sq | |
| Odontomachus rixosus F. Smith, 1857 | Md, Pl | |
| Odontomachus saevissimus F. Smith, 1858 | Lz, Rm | |
| Odontomachus schoedli Sorger & Zettel, 2011 | Lz | |
| Odontomachus scifictus Sorger & Zettel, 2011 | Cm | |
| Odontomachus simillimus F. Smith, 1858 | W | |
| Odontoponera denticulata Wheeler & Chapman, 1925 | W | |
| Oecophylla smaragdina (Fabricius, 1775) | W | |
| Overbeckia subclavata Viehmeyer, 1916a | MCZ – new record | |
| Pachycondyla annamita (Andre, 1892) | Lz | |
| Pachycondyla chinensis (Emery, 1895b) | Lz | |
| Pachycondyla claudata (Menozzi, 1926) | Lz, Sm | |
| Pachycondyla croceicornis (Emery, 1900a) | ||
| Pachycondyla darwinii indica (Emery, 1899) | Lz, Ng | |
| Pachycondyla glabripes (Emery, 1893c) | Md | |
| Pachycondyla javana (Mayr 1867) | ||
| Pachycondyla leeuwenhoeki (Forel, 1886a) | Ng | MCZ – new record |
| Pachycondyla luteipes (Mayr, 1862) | Lz, Ng, Pl | |
| Pachycondyla melancholica F. Smith, 1865 | ||
| Pachycondyla myropola (Menozzi, 1925) | Lz | |
| Pachycondyla obscurans (Walker, 1859) | Lz | |
| Pachycondyla stigma (Fabricius, 1804) | Bs, Lz, Md, Mr | |
| Pachycondyla testacea (Emery, 1893a) | Lz | A/G – new record |
| Pachycondyla tridentata F. Smith, 1858 | Jo, Tw | |
| Pachycondyla williamsi (Wheeler & Chapman, 1925) | Lz | |
| Paraparatrechina iridescens (Donisthorpe, 1942) | Lz, Pl | |
| Paratopula ceylonica (Emery, 1901) | ||
| Paratopula macta Bolton, 1988 | Lz | |
| Paratopula sumatrensis (Forel, 1913) | Lz | |
| Paratrechina longicornis (Latreille, 1802) | W | |
| Pheidole aglae Forel, 1913 | Lz | |
| Pheidole aristotelis Forel, 1911a | Ng, Pl | |
| Pheidole cariniceps Eguchi, 2001a | Lz | A/G – new record |
| Pheidole clypeocornis Eguchi, 2001a | Lz | |
| Pheidole fantasia Chapman, 1963 | Lz, Ng | |
| Pheidole fervens F. Smith, 1858 | Lz, Ng | |
| Pheidole guineensis Fabricius, 1793 | Pl | |
| Pheidole hortensis Forel, 1913 | Lz, Sm | |
| Pheidole inscrobiculata Viehmeyer, 1916a | Lz | |
| Pheidole jacobsoni Forel, 1911b | Lz, Md, Sm | |
| Pheidole longipes (F. Smith, 1857) | Md | |
| Pheidole maculifrons Wheeler, 1929d | Lz | |
| Pheidole makilingi Viehmeyer, 1916b | Lz | |
| Pheidole megacephala (Fabricius, 1793) | ||
| Pheidole plagiaria palawanica Stitz, 1925 | Ly, Pl | |
| Pheidole quadricuspis Emery, 1900a | Lz | |
| Pheidole sarawakana Forel, 1911a | Lz | |
| Pheidole sauberi Forel, 1905 | Lz | |
| Pheidole sayapensis Eguchi, 2001a | Lz | |
| Pheidole simoni Emery, 1893c | Lz | |
| Pheidole tjibodana Forel, 1905 | Lz | |
| Pheidologeton affinis (Jerdon, 1851) | Md, Pl | |
| Pheidologeton diversus (Jerdon, 1851) | Lz | |
| Pheidologeton diversus macgregori Wheeler, 1929 | Sm | |
| Pheidologeton diversus philippinus Wheeler, 1929 | Cb, Lz, Ng | |
| Pheidologeton diversus tenuirugosus Wheeler, 1929 | Lz | |
| Pheidologeton diversus williamsi Wheeler, 1929 | Lz, Md | |
| Pheidologeton maccus Wheeler, 1929d | Lz, Sm | |
| Pheidologeton pygmaeus albipes Emery, 1893c | Lz | |
| Pheidologeton silvestrii Wheeler, 1929d | Lz | |
| Philidris myrmecodiae (Emery, 1887a) | Lz | |
| Platythyrea bidentata Brown, 1975 | Ng | |
| Platythyrea inermis Forel, 1910 | Lz, Ng, Sm | |
| Platythyrea parallela (F. Smith, 1859) | Lz, Md, Ng, Rm | |
| Platythyrea quadridenta Donisthorpe, 1941b | Pl | |
| Polyrhachis abdominalis F. Smith, 1858 | Lz, Pl | |
| Polyrhachis aculeata Mayr, 1879 | Ng | |
| Polyrhachis aequalis Forel, 1910 | Lz, Ng, Pl | |
| Polyrhachis arcuata (Le Guillou, 1842) | ||
| Polyrhachis armata (Le Guillou, 1842) | Lz, Md, Ng, Pl | |
| Polyrhachis armata defensa F. Smith, 1857 | Pl | |
| Polyrhachis aureovestita Donisthorpe, 1937b | Md | |
| Polyrhachis baca Sorger & Zettel, 2010 | Lz | |
| Polyrhachis bakeri Viehmeyer, 1916b | Lz | |
| Polyrhachis banghaasi Viehmeyer, 1922 | ||
| Polyrhachis bellicosa F. Smith, 1859 | Lz, Ng | |
| Polyrhachis bicolor F. Smith, 1858 | Lz, Pl | |
| Polyrhachis bicolor aurinasis Forel, 1901b | ||
| Polyrhachis bicolor exflavicornis Baltazar, 1966 | ||
| Polyrhachis bicolor fumata Stitz, 1925 | Lz | |
| Polyrhachis bihamata (Drury, 1773) | Lz, Md, Ng | |
| Polyrhachis calypso Forel, 1911c | Tw | |
| Polyrhachis carbonaria F. Smith, 1857 | Pl | |
| Polyrhachis chapmani Kohout, 2006b | Ng | |
| Polyrhachis creusa Emery, 1897b | Ng | |
| Polyrhachis cryptoceroides Emery, 1887a | Lz | |
| Polyrhachis cyaniventris F. Smith, 1858 | Cb, Ct, Ly, Lz, Mr, Sm | |
| Polyrhachis diana Wheeler, 1909 | Md | |
| Polyrhachis dives F. Smith, 1857 | Lz, Pl | |
| Polyrhachis empesoi Kohout, 2006b | Ng | |
| Polyrhachis esuriens Emery, 1897b | Ng | |
| Polyrhachis etheli Chapman, 1963 | Ng | |
| Polyrhachis exotica Kohout, 1987 | Md | |
| Polyrhachis follicula Menozzi, 1926 | Lz | |
| Polyrhachis fruhstorferi Emery, 1898 | ||
| Polyrhachis hector F. Smith, 1857 | Ng | |
| Polyrhachis hippomanes boettcheri Stitz, 1925 | Lz, Md, Ng | |
| Polyrhachis ignota Kohout, 1987 | Lz | |
| Polyrhachis illaudata Walker, 1859 | Lz, Pl | |
| Polyrhachis inermis F. Smith, 1858 | Ng, Pl | |
| Polyrhachis javanica Mayr, 1867 | ||
| Polyrhachis lama Kohout, 1994 | Lz | |
| Polyrhachis magnifica Menozzi, 1926 | Lz, Ms, Ng, Sy, Tb | |
| Polyrhachis manni Kohout, 2008 | ||
| Polyrhachis marginata F. Smith, 1859 | ||
| Polyrhachis mindanaensis Emery, 1923 | Lz, Md | |
| Polyrhachis mitrata Menozzi, 1932 | Md | |
| Polyrhachis mucronata F. Smith, 1859 | ||
| Polyrhachis muelleri Forel, 1893a | Md, Ng | |
| Polyrhachis murina Emery, 1893b | Jo, Lz, Sm | |
| Polyrhachis neptunus F. Smith, 1865 | Lz | |
| Polyrhachis nigripes Emery, 1897b | Lz | |
| Polyrhachis nigropilosa Mayr, 1872 | Ng, Pl | |
| Polyrhachis nigropilosa polluta Menozzi, 1926 | Md | |
| Polyrhachis noesaensis Forel, 1915 | Ng, Pl, Si | |
| Polyrhachis obesior Viehmeyer, 1916a | ||
| Polyrhachis oedocantha Wheeler, 1919a | Ng | |
| Polyrhachis olybria Forel, 1912b | Lz, Md, Ng | |
| Polyrhachis orpheus Forel, 1911b | ||
| Polyrhachis osiris Bolton, 1975 | Lz, Ng | |
| Polyrhachis parabiotica Chapman, 1963 | Lz, Ng | |
| Polyrhachis pellita Menozzi, 1922 | Lz, Ng, Sm | |
| Polyrhachis phalerata Menozzi, 1926 | Lz | |
| Polyrhachis philippinensis F. Smith, 1858 | Lz | |
| Polyrhachis pirata Sorger and Zettel, 2009 | Lz, Mr, Sm | |
| Polyrhachis pressa Mayr, 1862 | Md | |
| Polyrhachis pubescens Mayr, 1879 | Lz | |
| Polyrhachis rixosa F. Smith, 1858 | Ng | |
| Polyrhachis rufipes F. Smith, 1858 | Lz | |
| Polyrhachis saevissima F. Smith, 1860a | Lz, Md, Ng, Pl | |
| Polyrhachis saevissima argentea Mayr, 1862 | Lz, Pl | |
| Polyrhachis saigonensis Forel, 1886b | Lz | |
| Polyrhachis scabra Kohout, 1987 | Md | |
| Polyrhachis schang Forel, 1879 | Ly, Sm | |
| Polyrhachis sculpturata F. Smith, 1860a | Lz, Pl | |
| Polyrhachis sculpturata javaniana Santschi, 1928b | Pl | |
| Polyrhachis semiinermis Donisthorpe, 1941c | Lz, Md | |
| Polyrhachis sexspinosa (Latreille, 1802) | Sm | |
| Polyrhachis solivaga Menozzi, 1926 | Lz | |
| Polyrhachis striata Mayr, 1862 | Pl | |
| Polyrhachis thrinax Roger, 1863 | Lz | |
| Polyrhachis thrinax castanea Stitz, 1925 | Lz | |
| Polyrhachis thrinax overbecki Dorow, 1995 | ||
| Polyrhachis tragos Stitz, 1925 | Lz, Md | |
| Polyrhachis tubifex Karavaiev, 1926 | Lz | |
| Polyrhachis venus Forel, 1893a | Lz, Md | |
| Polyrhachis villipes F. Smith, 1857 | Md | |
| Polyrhachis vindex F. Smith, 1857 | Lz, Ng | |
| Polyrhachis ypsilon Emery, 1887a | Lz, Ng | |
| Polyrhachis zopyra F. Smith, 1861 | Lz, Ng | |
| Ponera chapmani Taylor, 1967 | Lz, Md, Sm | |
| Ponera oreas (Wheeler, 1933) | Ng | |
| Prionopelta kraepelini Forel, 1905 | Lz, Ng, Sm, Pl | UPLB |
| Pristomyrmex bicolor Emery, 1900b | Lz, Pl | |
| Pristomyrmex brevispinosus Emery, 1887b | Md | |
| Pristomyrmex cebuensis Zettel, 2007 | Cb | |
| Pristomyrmex collinus Wang, 2003 | Lz, Md, Ng, Pn, Tb | |
| Pristomyrmex costatus Wang, 2003 | Md | |
| Pristomyrmex curvulus Wang, 2003 | Ng | |
| Pristomyrmex distinguendus Zettel, 2006 | Ly, Lz | |
| Pristomyrmex divisus Wang, 2003 | Ng | |
| Pristomyrmex flatus Wang, 2003 | Lz | |
| Pristomyrmex hirsutus Wang, 2003 | Md | |
| Pristomyrmex levigatus Emery, 1897a | Lz | |
| Pristomyrmex longispinus Wang, 2003 | Ng | |
| Pristomyrmex picteti Emery, 1893b | Ly, Lz, Md, Ng, Pl | |
| Pristomyrmex punctatus (F. Smith, 1860b) | Ly, Md, Mq, Mr, Pc, Sm | |
| Pristomyrmex quadridens Emery, 1897a | Ly | |
| Pristomyrmex rugosus Zettel, 2006 | Ly | |
| Pristomyrmex schoedli Zettel, 2006 | Ly | |
| Pristomyrmex simplex Wang, 2003 | Lz | |
| Probolomyrmex dammermani Wheeler, 1928 | Ng | MCZ – new record |
| Proceratium papuanum Emery, 1897a | Lz | A/G – new record |
| Pseudolasius typhlops Wheeler, 1935b | Lz, Pl | |
| Recurvidris nigrans Zettel, 2008 | Ng | |
| Rhoptromyrmex wroughtonii Forel, 1902a | Lz | MCZ, A/G |
| Rhytidoponera araneoides (Le Guillou, 1842) | Ng | BPBM– new record |
| Rhytidoponera croesus Emery 1901a | Lz | |
| Romblonella opaca (F. Smith, 1861) | Pl, Rm, Rr | |
| Simopone chapmani Taylor, 1966 | Ng | |
| Solenopsis geminata (Fabricius, 1804) | W | |
| Stigmatomma luzonicum Wheeler & Chapman, 1925 | Lz | |
| Stigmatomma reclinatum (Mayr, 1879) | Lz, Pl | |
| Stigmatomma rothneyi Forel, 1900c | Lz | |
| Strumigenys arrogantia Bolton, 2000 | Lz, Pl | |
| Strumigenys chapmani Brown, 1954 | Lz, Ng | |
| Strumigenys dohertyi Emery, 1897a | Lz | |
| Strumigenys emmae (Emery, 1890) | Lz | |
| Strumigenys esrossi Brown, 1957 | Lz | |
| Strumigenys godeffroyi geococci Calilung, 2000 | Lz | |
| Strumigenys godeffroyi Mayr, 1866a | Lz, Md, Pl | |
| Strumigenys imantodes Bolton, 2000 | Lz | |
| Strumigenys indigatrixWheeler, 1919a | Pl | |
| Strumigenys inhonesta Bolton, 2000 | Lz | |
| Strumigenys jacobsoni Menozzi, 1939 | Pl | |
| Strumigenys juliae Forel, 1905 | Pl | |
| Strumigenys karawajewi Brown, 1948 | Lz | |
| Strumigenys koningsbergeri Forel, 1905 | Lz, Pl | |
| Strumigenys lewisi Cameron, 1886 | Lz, Plw | A/G – new record |
| Strumigenys mirifica Bolton, 2000 | Lz | |
| Strumigenys mitis (Brown, 2000) | Lz | |
| Strumigenys mododonta Bolton, 2000 | Lz | |
| Strumigenys morphica Bolton, 2000 | Lz | A/G – new record |
| Strumigenys pedunculata (Brown, 1953) | Lz, Ng | |
| Strumigenys perplexa (F. Smith, 1876) | Lz | A/G – new record |
| Strumigenys phytibia Brown, 1957 | ||
| Strumigenys rantan Bolton, 2000 | Md | |
| Strumigenys rogeri Emery, 1890 | ||
| Strumigenys scylla (Bolton, 2000) | Lz | |
| Strumigenys serradens (Bolton, 2000) | Lz | |
| Strumigenys signeae Forel, 1905 | Pl | |
| Strumigenys synchysis Bolton, 2000 | Lz | A/G – new record |
| Strumigenys szalayi Emery, 1897a | Lz, Md, Mr, Ng | |
| Strumigenys tenitecta Bolton, 2000 | Lz | |
| Strumigenys uichancoi Brown, 1957 | ||
| Tapinoma emeryi (Ashmead, 1905) | Lz | SI |
| Tapinoma melanocephalum (Fabricius, 1793) | W | |
| Tapinoma philippinense Donisthorpe, 1942 | Lz | |
| Tapinoma williamsi (Wheeler, 1935b) | Lz | |
| Technomyrmex albipes (F. Smith, 1861) | Lz, Mr | |
| Technomyrmex difficilis Forel, 1892b | By, Lz, Md | |
| Technomyrmex elatior Forel, 1902b | Lz, Md | |
| Technomyrmex grandis Emery, 1887a | Bh, Ly, Md, Ng | |
| Technomyrmex kraepelini Forel, 1905 | Lz | |
| Technomyrmex pratensis (F. Smith, 1860b) | Md | |
| Technomyrmex sundaicus (Emery, 1900b) | Lz, Md, Sy | |
| Technomyrmex textor Forel, 1909 | Lz, Pl | |
| Technomyrmex vitiensis Mann, 1921 | Pl | |
| Technomyrmex wheeleri (Emery, 1913a) | Ly, Lz, Rm | |
| Tetramorium aspersum (F. Smith, 1865) | Lz, Md, Ng | |
| Tetramorium bicarinatum (Nylander, 1846) | W | |
| Tetramorium chapmani Bolton, 1977 | Ng | |
| Tetramorium cynicum Bolton, 1977 | Ng | |
| Tetramorium eleates Forel, 1913 | Lz | |
| Tetramorium guineense (Bernard, 1953) | Lz, Ng, Pl, Sm | |
| Tetramorium insolens (F. Smith, 1861) | Ng | |
| Tetramorium katypum (Bolton, 1976) | Cm, Lz, Md | |
| Tetramorium kheperra (Bolton, 1976) | Md | |
| Tetramorium khnum Bolton, 1977 | Ng | |
| Tetramorium kraepelini Forel, 1905 | Ng | |
| Tetramorium lanuginosum Mayr, 1870 | Mr, Ng | |
| Tetramorium laparum Bolton, 1977 | Ng | |
| Tetramorium manobo (Calilung, 2000) | Md | |
| Tetramorium obtusidens Viehmeyer, 1916a | ||
| Tetramorium pacificum Mayr, 1870 | Ly, Lz, Md, Ng, Rm | |
| Tetramorium parvispinum (Emery, 1893b) | Lz | |
| Tetramorium rinatum Bolton, 1977 | Ng | |
| Tetramorium simillimum (F. Smith, 1851) | Ng | |
| Tetramorium smithi Mayr, 1879 | ||
| Tetramorium tonganum Mayr, 1870 | ||
| Tetramorium tortuosum Roger, 1863 | ||
| Tetramorium walshi Forel 1890 | ||
| Tetramorium zypidum Bolton, 1977 | Ng | |
| Tetraponera allaborans (Walker, 1859) | W | |
| Tetraponera attenuata F. Smith, 1877 | Pl | |
| Tetraponera difficilis (Emery, 1900b) | Ly, Lz, Ng, Pl, Sm | |
| Tetraponera extenuata Ward, 2001 | Lz, Md | |
| Tetraponera inversinodis Ward, 2001 | Pl | |
| Tetraponera modesta (F. Smith, 1860b) | Ng, Pl, Rm, Sy, Tb | |
| Tetraponera nigra (Jerdon, 1851) | Pl | |
| Tetraponera nitida (F. Smith, 1860b) | Md, Ng, Pl | |
| Tetraponera pilosa (F. Smith, 1858) | Pl | |
| Vollenhovia ambitiosa Menozzi, 1925 | Lz, Md | |
| Vollenhovia banksi Forel, 1910 | Ng | |
| Vollenhovia cristata (Stitz, 1938) | Pl | |
| Vollenhovia luctuosa (Stitz, 1938) | Pl | |
| Vollenhovia oblonga dispar Forel, 1910 | Lz | |
| Vollenhovia soleaferrea Donisthorpe, 1942 | Lz | |
| Vombisidris philippina Zettel & Sorger, 2010a | Cb |
| Species | Distribution | Notes |
|---|---|---|
| Acanthomyrmex mindanao Moffett, 1986 | Md | |
| Acropyga acutiventris Roger, 1862 | Md | |
| Acropyga nipponensis Terayama, 1985 | Pl | |
| Acropyga pallida (Donisthorpe, 1938) | Lz | A/G – new record |
| Aenictus alticola Wheeler & Chapman (in Wheeler, 1930c) | Lz | |
| Aenictus bakeri Menozzi, 1925 | Md | |
| Aenictus camposi Wheeler & Chapman, 1925 | Lz, Ng | |
| Aenictus carolianus Zettel & Sorger, 2010b | Cb | |
| Aenictus ceylonicus (Mayr, 1866a) | Lz, Ng | |
| Aenictus gracilis Emery, 1893b | Lz, Md, Mr, Ng | |
| Aenictus laeviceps (F. Smith, 1857) | Ly, Lz, Md, Ng | |
| Aenictus luzoni Wheeler & Chapman, 1925 | Lz, Ng | |
| Aenictus nesiotis Wheeler & Chapman (in Wheeler, 1930c) | Lz, Ng, Pl | |
| Aenictus pangantihoni Zettel & Sorger, 2010b | Cm | |
| Aenictus philippinensis Chapman, 1963 | Ng | |
| Aenictus piercei Wheeler & Chapman (in Wheeler, 1930c) | Md, Ng | |
| Aenictus powersi Wheeler & Chapman (in Wheeler, 1930c) | Ng | |
| Aenictus rabori Chapman, 1963 | Ng | |
| Aenictus reyesi Chapman, 1963 | Ng | |
| Anillomyrma decamera (Emery, 1901b) | Lz | |
| Anochetus brevis Brown, 1978 | Md | |
| Anochetus cato Forel, 1901 | ||
| Anochetus graeffei Mayr, 1870 | Lz, Ms, Ng, Pl | |
| Anochetus incultus Brown, 1978 | Lz | |
| Anochetus isolatus Mann, 1919 | Lz, Ng | |
| Anochetus leyticus Zettel, 2012 | Ly | |
| Anochetus modicus Brown, 1978 | Lz, Ng | |
| Anochetus pangantihoni Zettel, 2012 | Ly | |
| Anochetus princeps Emery, 1884 | Lz | |
| Anochetus ruginotis Stitz, 1925 | Lz, Md | |
| Anochetus schoedli Zettel, 2012 | Lz | |
| Anochetus turneri Forel, 1900b | Lz | |
| Anochetus werneri Zettel, 2012 | Md | |
| Anomalomyrma helenae Borowiec, Schulz, Alpert & Baňař, 2011 | Pl | |
| Anoplolepis gracilipes (F. Smith, 1857) | W | |
| Basiceros chapmani (Taylor, 1990b) | Lz | |
| Basiceros philippinum (Brown & Kempf, 1960) | Lz, Ng | |
| Basiceros procerum (Emery, 1897a) | W | |
| Calyptomyrmex beccarii Emery, 1887b | Lz, Ng | |
| Calyptomyrmex loweryi Shattuck, 2011 | Lz, Md | |
| Camponotus albocinctus (Ashmead, 1905) | Lz | |
| Camponotus barbatus Roger, 1863 | ||
| Camponotus barbosus Baroni Urbani, 1971 | Lz, Md | |
| Camponotus bellus leucodiscus Wheeler, 1919a | Lz | |
| Camponotus carin Emery, 1889 | Lz | |
| Camponotus castanicola Donisthorpe, 1943b | Lz, Md | |
| Camponotus cinerascens (Fabricius, 1787) | ||
| Camponotus compressus (Fabricius, 1787) | Pl | |
| Camponotus corallinus (Roger, 1863) | Lz, Md | |
| Camponotus gigas (Latreille, 1802) | Lz | |
| Camponotus horrens Forel, 1910 | Lz | |
| Camponotus irritans pallidus (F. Smith, 1857) | Lz | |
| Camponotus japonicus Mayr, 1866b | Md | |
| Camponotus karawaiewi Menozzi, 1926 | Md | |
| Camponotus leonardi Emery, 1889 | Lz, Ng | |
| Camponotus leonardi gracilentus Stitz, 1925 | Lz | |
| Camponotus maculatus foveolatus Stitz, 1925 | ||
| Camponotus maculatus subnudus Stitz, 1925 | Lz, Pl | |
| Camponotus nicobarensis monticola Bingham, 1903 | Lz | |
| Camponotus nigricans Roger, 1863 | Jo, Lz | |
| Camponotus platypus Roger, 1863 | Lz, Md | |
| Camponotus pressipes Emery, 1893c | Md | |
| Camponotus quadrisectus (F. Smith, 1858) | Md, Pl | |
| Camponotus reticulatus jagori Stitz, 1925 | Lz | |
| Camponotus rothneyi makilingi Viehmeyer, 1916b | Lz | |
| Camponotus rufifemur obscurus Stitz, 1925 | Lz | |
| Camponotus solenobius Menozzi, 1926 | Md | |
| Camponotus trietericus Menozzi, 1926 | Lz | |
| Camponotus variegatus crassinodis Forel, 1892a | Ng | |
| Camponotus vitreus (F. Smith, 1860b) | W | |
| Cardiocondyla kagutsuchi Terayama, 1999 | By, Lz | |
| Cardiocondyla mauritanica (Forel, 1890) | Lz | |
| Cardiocondyla sima Wheeler, 1935b | Lz, Md | |
| Cardiocondyla tjibodana Karavaiev, 1935 | By, Lz | |
| Cardiocondyla wroughtonii (Forel, 1890) | Lz | |
| Carebara alperti Fernandez, 2010 | Ng | |
| Cataulacus catuvolcus Bolton, 1974 | Lz, Rm | |
| Cataulacus chapmani Bolton, 1974 | Lz, Ng | |
| Cataulacus setosus F. Smith, 1860b | Md | |
| Centromyrmex feae (Emery, 1889) | Bs, Lz | |
| Cerapachys bakeri (Wheeler & Chapman, 1925) | Bs | |
| Cerapachys biroi Forel, 1907 | ||
| Cerapachys crawleyi Wheeler, 1924b | Lz, Ng | |
| Cerapachys jacobsoni Forel, 1912a | Ng | |
| Cerapachys longitarsus (Mayr, 1879) | Lz | |
| Cerapachys luzuriagae (Wheeler & Chapman, 1925) | Ng | |
| Cerapachys muiri Wheeler & Chapman, 1925 | Lz, Md | |
| Cerapachys pruinosus Brown, 1975 | Ng | |
| Cerapachys rufithorax Wheeler & Chapman, 1925 | Lz, Ng | |
| Cerapachys sulcinodis Emery, 1889 | Lz, Ng | |
| Cerapachys suscitatus (Viehmeyer, 1913) | Ng | |
| Crematogaster ampullaris F. Smith, 1861 | Ly, Lz | |
| Crematogaster bakeri Menozzi, 1925 | Md | |
| Crematogaster bicolor imbellis Emery, 1893c | Lz, Ng | |
| Crematogaster brunnea ruginota Santschi, 1928a | ||
| Crematogaster crassicornis Emery, 1893c | Lz, Md | |
| Crematogaster cylindriceps Wheeler, 1927 | ||
| Crematogaster difformis F. Smith, 1857 | Lz | |
| Crematogaster inflata F. Smith, 1857 | Lz | |
| Crematogaster longiclava Emery, 1893c | Lz | |
| Crematogaster modiglianii Emery, 1900b | Md, Ng | |
| Crematogaster ochracea Mayr, 1862 | Lz, Ng | |
| Crematogaster onusta Stitz, 1925 | Pl, SC | |
| Crematogaster rogenhoferi Mayr, 1879 | Lz | |
| Crematogaster semperi Emery, 1893c | Lz | |
| Crematogaster simoni Emery, 1893c | Lz | |
| Crematogaster vitalisi Menozzi, 1925 | Md | |
| Dacetinops cirrosus Taylor, 1985 | Ly | HZW – new record |
| Diacamma baguiense Wheeler & Chapman, 1925 | Lz | |
| Diacamma palawanicum concentricum Wheeler & Chapman, 1925 | Pl | |
| Diacamma palawanicum Emery, 1900b | Pl | |
| Diacamma panayense Wheeler & Chapman, 1925 | Pn | |
| Diacamma rugosum (Le Guillou, 1842) | W | |
| Diacamma rugosum viridipurpureum Emery, 1893c | Lz | |
| Diacamma sericeiventre Stitz, 1925 | Pl | |
| Dilobocondyla chapmani rufobrunnea Wheeler, 1935b | Ng | |
| Dilobocondyla chapmani Wheeler, 1924a | Ng | |
| Discothyrea bryanti (Wheeler, 1917) | Lz | |
| Discothyrea clavicornis Emery, 1897a | Lz | |
| Dolichoderus affinis Emery, 1889 | ||
| Dolichoderus patens pubiventris Emery, 1900b | Pl | |
| Dolichoderus thoracicus (F. Smith, 1860a) | W | |
| Echinopla pallipes F. Smith, 1857 | Lz | |
| Echinopla pseudostriata Donisthorpe, 1943a | Lz | |
| Echinopla striata aciculata (F. Smith, 1858) | ||
| Echinopla striata F. Smith, 1857 | ||
| Echinopla vermiculata Emery, 1898 | Lz | |
| Euprenolepis negrosensis (Wheeler, 1930b) | Ng | |
| Forelophilus philippinensis Zettel & Zimmerman, 2007 | Lz, Md | |
| Forelophilus stefanschoedli Zettel & Zimmerman, 2007 | Ly, Lz, Md | |
| Gauromyrmex acanthinus (Karavaiev, 1935) | MCZ – new record | |
| Gesomyrmex luzonensis (Wheeler, 1916) | Lz, Ng | |
| Gnamptogenys binghamii (Forel, 1900d) | Lz, Ng | |
| Gnamptogenys bulbopila Lattke, 2004 | Md | |
| Gnamptogenys chapmani Brown, 1958 | Lz, Ng | |
| Gnamptogenys costata (Emery, 1889) | Lz, Md | |
| Gnamptogenys cribrata (Emery, 1900a) | Lz, Ng | |
| Gnamptogenys fistulosa Lattke, 2004 | Lz | |
| Gnamptogenys laevior (Forel, 1905) | Lz | |
| Gnamptogenys leiolabia Lattke, 2004 | Ng | |
| Gnamptogenys luzonensis (Wheeler, 1929d) | Lz, Ng | |
| Gnamptogenys menadensis (Mayr, 1887) | Lz | |
| Gnamptogenys posteropsis (Gregg, 1951) | Lz, Md, Ng | |
| Harpegnathos empesoi Chapman, 1963 | Md | |
| Harpegnathos macgregori Wheeler & Chapman, 1925 | Bi | |
| Harpegnathos medioniger Donisthorpe, 1942 | Lz | |
| Harpegnathos venator chapmani Donisthorpe, 1937 | Lz | |
| Hypoponera confinis javana (Forel, 1905) | Lz, Ng | |
| Hypoponera opaciceps (Mayr, 1887) | ||
| Hypoponera pruinosa (Emery, 1900a) | Lz | |
| Hypoponera punctatissima (Roger, 1859) | Lz | |
| Hypoponera ragusai (Emery, 1894) | Lz | |
| Hypoponera sabronae (Donisthorpe, 1941b) | ||
| Iridomyrmex anceps (Roger, 1863) | Mq | |
| Iridomyrmex angusticeps Forel, 1901 | Md | |
| Lepisiota aurea punctaticeps Wheeler, 1935b | Lz, Ng | |
| Lepisiota chapmani Wheeler, 1935b | Ng | |
| Leptanilla astylina Petersen, 1968 | Pl | |
| Leptogenys chinensis (Mayr, 1870) | Ng | |
| Leptogenys diminuta (F. Smith, 1857) | Lz, Md, Ng, Pl | |
| Leptogenys falcigera Roger, 1861 | Ng | |
| Leptogenys iridescens (F. Smith, 1857) | Pl | |
| Leptogenys maxillosa (F. Smith, 1858) | Pn | |
| Leptogenys mutabilis (F. Smith, 1861) | Pl | |
| Leptogenys peuqueti (Andre, 1887) | Lz, Ng | |
| Leptogenys pruinosa Forel, 1900d | Lz, Ng | |
| Leptogenys punctiventris (Mayr, 1879) | Lz, Ng | |
| Leptogenys varicosa (Stitz, 1925) | Pl | |
| Leptogenys watsoni Forel, 1900d | Lz, Ng, Pn | |
| Leptomyrmex fragilis (F. Smith, 1859) | Md | BPBM – new record |
| Liomyrmex gestroi (Emery, 1887b) | W | |
| Lophomyrmex bedoti Emery, 1893b | Pl | |
| Lordomyrma diwata Taylor, 2012 | Lz | |
| Lordomyrma emarginata Taylor, 2012 | Lz | |
| Lordomyrma idianale Taylor, 2012 | Lz | |
| Lordomyrma limatula Taylor, 2012 | Ly | |
| Mayriella transfuga Baroni Urbani, 1977a | Lz | A/G – new record |
| Meranoplus biliran Schödl, 1998 | Bi | |
| Metapone bakeri Wheeler, 1916 | Lz | |
| Metapone gracilis Wheeler, 1935b | Md | |
| Monomorium banksi Forel, 1910 | Ng | |
| Monomorium destructor (Jerdon, 1851) | Lz | |
| Monomorium floricola (Jerdon, 1851) | Cm, Lz, Md, Tb | |
| Monomorium orientale Mayr, 1879 | ||
| Monomorium pharaonis (Linnaeus, 1758) | W | |
| Myopias bidens polita (Stitz, 1925) | Ng, Pl | |
| Myopias breviloba (Wheeler, 1919a) | Bs | |
| Myopias lobosa Willey & Brown, 1983 | Lz | |
| Myopias modiglianii (Emery 1900b) | Ng | |
| Myopias philippinensis (Menozzi, 1925) | Lz, Md | |
| Myopopone castanea (F. Smith 1860b) | Ly, Lz, Md, Pl | |
| Myrmicaria aphidicola Calilung, 2000 | Md | |
| Myrmicaria brunnea subcarinata (F. Smith, 1857) | W | |
| Myrmoteras glabrum Zettel & Sorger, 2011 | Cm | |
| Myrmoteras insulcatum Moffett, 1985 | Lz, Ng | |
| Myrmoteras mcarthuri Zettel & Sorger, 2011 | Ly | |
| Myrmoteras williamsi Wheeler, 1919a | Lz, Ng | |
| Mystrium camillae Emery, 1889 | Lz, Pl | |
| Noonilla copiosa Petersen, 1968 | Pl | |
| Ochetellus glaber (Mayr, 1862) | Lz, Ng | MCZ – new record |
| Odontomachus alius Sorger & Zettel, 2011 | Bh, Bi, Cb, Ct, Ly, Sm | |
| Odontomachus banksi Forel, 1910 | Cb, Lz | |
| Odontomachus infandus F. Smith, 1858 | Lz, Mr, Ng, Rm | |
| Odontomachus malignus F. Smith, 1859 | Ba, Bh, Jo, Tw | |
| Odontomachus papuanus Emery, 1887b | Ng, Pl, Pn | |
| Odontomachus philippinus Emery, 1893b | Ng, Pn, Sq | |
| Odontomachus rixosus F. Smith, 1857 | Md, Pl | |
| Odontomachus saevissimus F. Smith, 1858 | Lz, Rm | |
| Odontomachus schoedli Sorger & Zettel, 2011 | Lz | |
| Odontomachus scifictus Sorger & Zettel, 2011 | Cm | |
| Odontomachus simillimus F. Smith, 1858 | W | |
| Odontoponera denticulata Wheeler & Chapman, 1925 | W | |
| Oecophylla smaragdina (Fabricius, 1775) | W | |
| Overbeckia subclavata Viehmeyer, 1916a | MCZ – new record | |
| Pachycondyla annamita (Andre, 1892) | Lz | |
| Pachycondyla chinensis (Emery, 1895b) | Lz | |
| Pachycondyla claudata (Menozzi, 1926) | Lz, Sm | |
| Pachycondyla croceicornis (Emery, 1900a) | ||
| Pachycondyla darwinii indica (Emery, 1899) | Lz, Ng | |
| Pachycondyla glabripes (Emery, 1893c) | Md | |
| Pachycondyla javana (Mayr 1867) | ||
| Pachycondyla leeuwenhoeki (Forel, 1886a) | Ng | MCZ – new record |
| Pachycondyla luteipes (Mayr, 1862) | Lz, Ng, Pl | |
| Pachycondyla melancholica F. Smith, 1865 | ||
| Pachycondyla myropola (Menozzi, 1925) | Lz | |
| Pachycondyla obscurans (Walker, 1859) | Lz | |
| Pachycondyla stigma (Fabricius, 1804) | Bs, Lz, Md, Mr | |
| Pachycondyla testacea (Emery, 1893a) | Lz | A/G – new record |
| Pachycondyla tridentata F. Smith, 1858 | Jo, Tw | |
| Pachycondyla williamsi (Wheeler & Chapman, 1925) | Lz | |
| Paraparatrechina iridescens (Donisthorpe, 1942) | Lz, Pl | |
| Paratopula ceylonica (Emery, 1901) | ||
| Paratopula macta Bolton, 1988 | Lz | |
| Paratopula sumatrensis (Forel, 1913) | Lz | |
| Paratrechina longicornis (Latreille, 1802) | W | |
| Pheidole aglae Forel, 1913 | Lz | |
| Pheidole aristotelis Forel, 1911a | Ng, Pl | |
| Pheidole cariniceps Eguchi, 2001a | Lz | A/G – new record |
| Pheidole clypeocornis Eguchi, 2001a | Lz | |
| Pheidole fantasia Chapman, 1963 | Lz, Ng | |
| Pheidole fervens F. Smith, 1858 | Lz, Ng | |
| Pheidole guineensis Fabricius, 1793 | Pl | |
| Pheidole hortensis Forel, 1913 | Lz, Sm | |
| Pheidole inscrobiculata Viehmeyer, 1916a | Lz | |
| Pheidole jacobsoni Forel, 1911b | Lz, Md, Sm | |
| Pheidole longipes (F. Smith, 1857) | Md | |
| Pheidole maculifrons Wheeler, 1929d | Lz | |
| Pheidole makilingi Viehmeyer, 1916b | Lz | |
| Pheidole megacephala (Fabricius, 1793) | ||
| Pheidole plagiaria palawanica Stitz, 1925 | Ly, Pl | |
| Pheidole quadricuspis Emery, 1900a | Lz | |
| Pheidole sarawakana Forel, 1911a | Lz | |
| Pheidole sauberi Forel, 1905 | Lz | |
| Pheidole sayapensis Eguchi, 2001a | Lz | |
| Pheidole simoni Emery, 1893c | Lz | |
| Pheidole tjibodana Forel, 1905 | Lz | |
| Pheidologeton affinis (Jerdon, 1851) | Md, Pl | |
| Pheidologeton diversus (Jerdon, 1851) | Lz | |
| Pheidologeton diversus macgregori Wheeler, 1929 | Sm | |
| Pheidologeton diversus philippinus Wheeler, 1929 | Cb, Lz, Ng | |
| Pheidologeton diversus tenuirugosus Wheeler, 1929 | Lz | |
| Pheidologeton diversus williamsi Wheeler, 1929 | Lz, Md | |
| Pheidologeton maccus Wheeler, 1929d | Lz, Sm | |
| Pheidologeton pygmaeus albipes Emery, 1893c | Lz | |
| Pheidologeton silvestrii Wheeler, 1929d | Lz | |
| Philidris myrmecodiae (Emery, 1887a) | Lz | |
| Platythyrea bidentata Brown, 1975 | Ng | |
| Platythyrea inermis Forel, 1910 | Lz, Ng, Sm | |
| Platythyrea parallela (F. Smith, 1859) | Lz, Md, Ng, Rm | |
| Platythyrea quadridenta Donisthorpe, 1941b | Pl | |
| Polyrhachis abdominalis F. Smith, 1858 | Lz, Pl | |
| Polyrhachis aculeata Mayr, 1879 | Ng | |
| Polyrhachis aequalis Forel, 1910 | Lz, Ng, Pl | |
| Polyrhachis arcuata (Le Guillou, 1842) | ||
| Polyrhachis armata (Le Guillou, 1842) | Lz, Md, Ng, Pl | |
| Polyrhachis armata defensa F. Smith, 1857 | Pl | |
| Polyrhachis aureovestita Donisthorpe, 1937b | Md | |
| Polyrhachis baca Sorger & Zettel, 2010 | Lz | |
| Polyrhachis bakeri Viehmeyer, 1916b | Lz | |
| Polyrhachis banghaasi Viehmeyer, 1922 | ||
| Polyrhachis bellicosa F. Smith, 1859 | Lz, Ng | |
| Polyrhachis bicolor F. Smith, 1858 | Lz, Pl | |
| Polyrhachis bicolor aurinasis Forel, 1901b | ||
| Polyrhachis bicolor exflavicornis Baltazar, 1966 | ||
| Polyrhachis bicolor fumata Stitz, 1925 | Lz | |
| Polyrhachis bihamata (Drury, 1773) | Lz, Md, Ng | |
| Polyrhachis calypso Forel, 1911c | Tw | |
| Polyrhachis carbonaria F. Smith, 1857 | Pl | |
| Polyrhachis chapmani Kohout, 2006b | Ng | |
| Polyrhachis creusa Emery, 1897b | Ng | |
| Polyrhachis cryptoceroides Emery, 1887a | Lz | |
| Polyrhachis cyaniventris F. Smith, 1858 | Cb, Ct, Ly, Lz, Mr, Sm | |
| Polyrhachis diana Wheeler, 1909 | Md | |
| Polyrhachis dives F. Smith, 1857 | Lz, Pl | |
| Polyrhachis empesoi Kohout, 2006b | Ng | |
| Polyrhachis esuriens Emery, 1897b | Ng | |
| Polyrhachis etheli Chapman, 1963 | Ng | |
| Polyrhachis exotica Kohout, 1987 | Md | |
| Polyrhachis follicula Menozzi, 1926 | Lz | |
| Polyrhachis fruhstorferi Emery, 1898 | ||
| Polyrhachis hector F. Smith, 1857 | Ng | |
| Polyrhachis hippomanes boettcheri Stitz, 1925 | Lz, Md, Ng | |
| Polyrhachis ignota Kohout, 1987 | Lz | |
| Polyrhachis illaudata Walker, 1859 | Lz, Pl | |
| Polyrhachis inermis F. Smith, 1858 | Ng, Pl | |
| Polyrhachis javanica Mayr, 1867 | ||
| Polyrhachis lama Kohout, 1994 | Lz | |
| Polyrhachis magnifica Menozzi, 1926 | Lz, Ms, Ng, Sy, Tb | |
| Polyrhachis manni Kohout, 2008 | ||
| Polyrhachis marginata F. Smith, 1859 | ||
| Polyrhachis mindanaensis Emery, 1923 | Lz, Md | |
| Polyrhachis mitrata Menozzi, 1932 | Md | |
| Polyrhachis mucronata F. Smith, 1859 | ||
| Polyrhachis muelleri Forel, 1893a | Md, Ng | |
| Polyrhachis murina Emery, 1893b | Jo, Lz, Sm | |
| Polyrhachis neptunus F. Smith, 1865 | Lz | |
| Polyrhachis nigripes Emery, 1897b | Lz | |
| Polyrhachis nigropilosa Mayr, 1872 | Ng, Pl | |
| Polyrhachis nigropilosa polluta Menozzi, 1926 | Md | |
| Polyrhachis noesaensis Forel, 1915 | Ng, Pl, Si | |
| Polyrhachis obesior Viehmeyer, 1916a | ||
| Polyrhachis oedocantha Wheeler, 1919a | Ng | |
| Polyrhachis olybria Forel, 1912b | Lz, Md, Ng | |
| Polyrhachis orpheus Forel, 1911b | ||
| Polyrhachis osiris Bolton, 1975 | Lz, Ng | |
| Polyrhachis parabiotica Chapman, 1963 | Lz, Ng | |
| Polyrhachis pellita Menozzi, 1922 | Lz, Ng, Sm | |
| Polyrhachis phalerata Menozzi, 1926 | Lz | |
| Polyrhachis philippinensis F. Smith, 1858 | Lz | |
| Polyrhachis pirata Sorger and Zettel, 2009 | Lz, Mr, Sm | |
| Polyrhachis pressa Mayr, 1862 | Md | |
| Polyrhachis pubescens Mayr, 1879 | Lz | |
| Polyrhachis rixosa F. Smith, 1858 | Ng | |
| Polyrhachis rufipes F. Smith, 1858 | Lz | |
| Polyrhachis saevissima F. Smith, 1860a | Lz, Md, Ng, Pl | |
| Polyrhachis saevissima argentea Mayr, 1862 | Lz, Pl | |
| Polyrhachis saigonensis Forel, 1886b | Lz | |
| Polyrhachis scabra Kohout, 1987 | Md | |
| Polyrhachis schang Forel, 1879 | Ly, Sm | |
| Polyrhachis sculpturata F. Smith, 1860a | Lz, Pl | |
| Polyrhachis sculpturata javaniana Santschi, 1928b | Pl | |
| Polyrhachis semiinermis Donisthorpe, 1941c | Lz, Md | |
| Polyrhachis sexspinosa (Latreille, 1802) | Sm | |
| Polyrhachis solivaga Menozzi, 1926 | Lz | |
| Polyrhachis striata Mayr, 1862 | Pl | |
| Polyrhachis thrinax Roger, 1863 | Lz | |
| Polyrhachis thrinax castanea Stitz, 1925 | Lz | |
| Polyrhachis thrinax overbecki Dorow, 1995 | ||
| Polyrhachis tragos Stitz, 1925 | Lz, Md | |
| Polyrhachis tubifex Karavaiev, 1926 | Lz | |
| Polyrhachis venus Forel, 1893a | Lz, Md | |
| Polyrhachis villipes F. Smith, 1857 | Md | |
| Polyrhachis vindex F. Smith, 1857 | Lz, Ng | |
| Polyrhachis ypsilon Emery, 1887a | Lz, Ng | |
| Polyrhachis zopyra F. Smith, 1861 | Lz, Ng | |
| Ponera chapmani Taylor, 1967 | Lz, Md, Sm | |
| Ponera oreas (Wheeler, 1933) | Ng | |
| Prionopelta kraepelini Forel, 1905 | Lz, Ng, Sm, Pl | UPLB |
| Pristomyrmex bicolor Emery, 1900b | Lz, Pl | |
| Pristomyrmex brevispinosus Emery, 1887b | Md | |
| Pristomyrmex cebuensis Zettel, 2007 | Cb | |
| Pristomyrmex collinus Wang, 2003 | Lz, Md, Ng, Pn, Tb | |
| Pristomyrmex costatus Wang, 2003 | Md | |
| Pristomyrmex curvulus Wang, 2003 | Ng | |
| Pristomyrmex distinguendus Zettel, 2006 | Ly, Lz | |
| Pristomyrmex divisus Wang, 2003 | Ng | |
| Pristomyrmex flatus Wang, 2003 | Lz | |
| Pristomyrmex hirsutus Wang, 2003 | Md | |
| Pristomyrmex levigatus Emery, 1897a | Lz | |
| Pristomyrmex longispinus Wang, 2003 | Ng | |
| Pristomyrmex picteti Emery, 1893b | Ly, Lz, Md, Ng, Pl | |
| Pristomyrmex punctatus (F. Smith, 1860b) | Ly, Md, Mq, Mr, Pc, Sm | |
| Pristomyrmex quadridens Emery, 1897a | Ly | |
| Pristomyrmex rugosus Zettel, 2006 | Ly | |
| Pristomyrmex schoedli Zettel, 2006 | Ly | |
| Pristomyrmex simplex Wang, 2003 | Lz | |
| Probolomyrmex dammermani Wheeler, 1928 | Ng | MCZ – new record |
| Proceratium papuanum Emery, 1897a | Lz | A/G – new record |
| Pseudolasius typhlops Wheeler, 1935b | Lz, Pl | |
| Recurvidris nigrans Zettel, 2008 | Ng | |
| Rhoptromyrmex wroughtonii Forel, 1902a | Lz | MCZ, A/G |
| Rhytidoponera araneoides (Le Guillou, 1842) | Ng | BPBM– new record |
| Rhytidoponera croesus Emery 1901a | Lz | |
| Romblonella opaca (F. Smith, 1861) | Pl, Rm, Rr | |
| Simopone chapmani Taylor, 1966 | Ng | |
| Solenopsis geminata (Fabricius, 1804) | W | |
| Stigmatomma luzonicum Wheeler & Chapman, 1925 | Lz | |
| Stigmatomma reclinatum (Mayr, 1879) | Lz, Pl | |
| Stigmatomma rothneyi Forel, 1900c | Lz | |
| Strumigenys arrogantia Bolton, 2000 | Lz, Pl | |
| Strumigenys chapmani Brown, 1954 | Lz, Ng | |
| Strumigenys dohertyi Emery, 1897a | Lz | |
| Strumigenys emmae (Emery, 1890) | Lz | |
| Strumigenys esrossi Brown, 1957 | Lz | |
| Strumigenys godeffroyi geococci Calilung, 2000 | Lz | |
| Strumigenys godeffroyi Mayr, 1866a | Lz, Md, Pl | |
| Strumigenys imantodes Bolton, 2000 | Lz | |
| Strumigenys indigatrixWheeler, 1919a | Pl | |
| Strumigenys inhonesta Bolton, 2000 | Lz | |
| Strumigenys jacobsoni Menozzi, 1939 | Pl | |
| Strumigenys juliae Forel, 1905 | Pl | |
| Strumigenys karawajewi Brown, 1948 | Lz | |
| Strumigenys koningsbergeri Forel, 1905 | Lz, Pl | |
| Strumigenys lewisi Cameron, 1886 | Lz, Plw | A/G – new record |
| Strumigenys mirifica Bolton, 2000 | Lz | |
| Strumigenys mitis (Brown, 2000) | Lz | |
| Strumigenys mododonta Bolton, 2000 | Lz | |
| Strumigenys morphica Bolton, 2000 | Lz | A/G – new record |
| Strumigenys pedunculata (Brown, 1953) | Lz, Ng | |
| Strumigenys perplexa (F. Smith, 1876) | Lz | A/G – new record |
| Strumigenys phytibia Brown, 1957 | ||
| Strumigenys rantan Bolton, 2000 | Md | |
| Strumigenys rogeri Emery, 1890 | ||
| Strumigenys scylla (Bolton, 2000) | Lz | |
| Strumigenys serradens (Bolton, 2000) | Lz | |
| Strumigenys signeae Forel, 1905 | Pl | |
| Strumigenys synchysis Bolton, 2000 | Lz | A/G – new record |
| Strumigenys szalayi Emery, 1897a | Lz, Md, Mr, Ng | |
| Strumigenys tenitecta Bolton, 2000 | Lz | |
| Strumigenys uichancoi Brown, 1957 | ||
| Tapinoma emeryi (Ashmead, 1905) | Lz | SI |
| Tapinoma melanocephalum (Fabricius, 1793) | W | |
| Tapinoma philippinense Donisthorpe, 1942 | Lz | |
| Tapinoma williamsi (Wheeler, 1935b) | Lz | |
| Technomyrmex albipes (F. Smith, 1861) | Lz, Mr | |
| Technomyrmex difficilis Forel, 1892b | By, Lz, Md | |
| Technomyrmex elatior Forel, 1902b | Lz, Md | |
| Technomyrmex grandis Emery, 1887a | Bh, Ly, Md, Ng | |
| Technomyrmex kraepelini Forel, 1905 | Lz | |
| Technomyrmex pratensis (F. Smith, 1860b) | Md | |
| Technomyrmex sundaicus (Emery, 1900b) | Lz, Md, Sy | |
| Technomyrmex textor Forel, 1909 | Lz, Pl | |
| Technomyrmex vitiensis Mann, 1921 | Pl | |
| Technomyrmex wheeleri (Emery, 1913a) | Ly, Lz, Rm | |
| Tetramorium aspersum (F. Smith, 1865) | Lz, Md, Ng | |
| Tetramorium bicarinatum (Nylander, 1846) | W | |
| Tetramorium chapmani Bolton, 1977 | Ng | |
| Tetramorium cynicum Bolton, 1977 | Ng | |
| Tetramorium eleates Forel, 1913 | Lz | |
| Tetramorium guineense (Bernard, 1953) | Lz, Ng, Pl, Sm | |
| Tetramorium insolens (F. Smith, 1861) | Ng | |
| Tetramorium katypum (Bolton, 1976) | Cm, Lz, Md | |
| Tetramorium kheperra (Bolton, 1976) | Md | |
| Tetramorium khnum Bolton, 1977 | Ng | |
| Tetramorium kraepelini Forel, 1905 | Ng | |
| Tetramorium lanuginosum Mayr, 1870 | Mr, Ng | |
| Tetramorium laparum Bolton, 1977 | Ng | |
| Tetramorium manobo (Calilung, 2000) | Md | |
| Tetramorium obtusidens Viehmeyer, 1916a | ||
| Tetramorium pacificum Mayr, 1870 | Ly, Lz, Md, Ng, Rm | |
| Tetramorium parvispinum (Emery, 1893b) | Lz | |
| Tetramorium rinatum Bolton, 1977 | Ng | |
| Tetramorium simillimum (F. Smith, 1851) | Ng | |
| Tetramorium smithi Mayr, 1879 | ||
| Tetramorium tonganum Mayr, 1870 | ||
| Tetramorium tortuosum Roger, 1863 | ||
| Tetramorium walshi Forel 1890 | ||
| Tetramorium zypidum Bolton, 1977 | Ng | |
| Tetraponera allaborans (Walker, 1859) | W | |
| Tetraponera attenuata F. Smith, 1877 | Pl | |
| Tetraponera difficilis (Emery, 1900b) | Ly, Lz, Ng, Pl, Sm | |
| Tetraponera extenuata Ward, 2001 | Lz, Md | |
| Tetraponera inversinodis Ward, 2001 | Pl | |
| Tetraponera modesta (F. Smith, 1860b) | Ng, Pl, Rm, Sy, Tb | |
| Tetraponera nigra (Jerdon, 1851) | Pl | |
| Tetraponera nitida (F. Smith, 1860b) | Md, Ng, Pl | |
| Tetraponera pilosa (F. Smith, 1858) | Pl | |
| Vollenhovia ambitiosa Menozzi, 1925 | Lz, Md | |
| Vollenhovia banksi Forel, 1910 | Ng | |
| Vollenhovia cristata (Stitz, 1938) | Pl | |
| Vollenhovia luctuosa (Stitz, 1938) | Pl | |
| Vollenhovia oblonga dispar Forel, 1910 | Lz | |
| Vollenhovia soleaferrea Donisthorpe, 1942 | Lz | |
| Vombisidris philippina Zettel & Sorger, 2010a | Cb |
Key to Notes:
A/G = Alpert and General Collection
BPBM = Bernice P. Bishop Museum Collection
HZW = Herbert Zettel (Vienna) Collection
MCZ = Museum of Comparative Zoology Collection
UPLB = University of the Philippines at Los Baños Museum of Natural History Collection
SI = Smithsonian Institution Museum Collection
Key to Island Abbreviations:
Ba = Bantayan
Bh = Bohol
Bi = Biliran
Bs = Basilan
By = Bayagnan
Cb = Cebu
Cm = Camiguin
Ct= Catanduanes
Jo = Jolo
Ly = Leyte
Lz = Luzon
Md = Mindanao
Mq = Marinduque
Mr = Mindoro
Ms = Masbate
Ng = Negros
Pc = Pacijan
Pl = Palawan
Pn = Panay
Po = Polillo
Rm = Romblon
Rr = Rapu-rapu
SC = Sta. Cruz
Si = Sibutu
Sm = Samar
Sy = Sibuyan
Tb = Tablas
Tw = Tawi-tawi
W = Widespread