(C) 2013 Menno Reemer. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
With 552 species group names available (excluding misspellings), the Microdontinae constitute the smallest of the three subfamilies of Syrphidae. Paradoxically, this subfamily is taxonomically the least organized of the three: 388 species names were previously classified in a single genus, Microdon Meigen, 1803. The present paper introduces a new generic classification of the Microdontinae, relying partly on the results of phylogenetic analyses of morphological and molecular data as published in other papers, and partly on examination of primary type specimens of 347 taxa, plus additional material, and original descriptions. A total number of 67 genus group names (excluding misspellings) are evaluated, redescribed, diagnosed and discussed, with several implications for their taxonomic status. Of these, 43 names are considered as valid genera, 7 as subgenera, 17 as synonyms. Two generic names (Ceratoconcha Simroth, 1907, Nothomicrodon Wheeler, 1924) are left unplaced, because they are known from immature stages only and cannot be reliably associated with taxa known from adults. The following 10 new genera are described by Reemer: Domodon, Heliodon, Laetodon, Menidon, Mermerizon, Metadon, Peradon, Piruwa, Sulcodon and Thompsodon. A key to all genera, subgenera and species groups is given. A total number of 26 new species are described in the following genera: Archimicrodon Hull, 1945, Ceratrichomyia Séguy, 1951, Domodon, Furcantenna Cheng, 2008, Heliodon, Indascia Keiser, 1958, Kryptopyga Hull, 1944, Masarygus Brèthes. 1908, Mermerizon, Metadon, Microdon, Paramixogaster Brunetti, 1923, Piruwa, Pseudomicrodon Hull, 1937, Rhopalosyrphus Giglio-Tos, 1891, and Thompsodon. New lectotypes are designated for Ceratrichomyia behara Séguy, 1951 and Microdon iheringi Bezzi, 1910. A total number of 267 new combinations of species and genera are proposed. New synonyms are proposed for 19 species group names. Three replacement names are introduced for primary and secondary junior homonyms: Microdon shirakii nom. n. (= Microdon tuberculatus Shiraki, 1968, primary homonym of Microdon tuberculatus de Meijere, 1913), Paramixogaster brunettii nom. n. (= Mixogaster vespiformis Brunetti, 1913, secondary homonym of Microdon vespiformis de Meijere, 1908), Paramixogaster sacki nom. n. (= Myxogaster variegata Sack, 1922, secondary homonym of Ceratophya variegata Walker, 1852). An attempt is made to classify all available species names into (sub)genera and species groups. The resulting classification comprises 454 valid species and 98 synonyms (excluding misspellings), of which 17 valid names and three synonyms are left unplaced. The paper concludes with a discussion on diagnostic characters of Microdontinae.
Key, revision, new genera, new species, new synonyms, new combinations, catalogue
The Microdontinae (Diptera: Syrphidae) are found on all continents except Antarctica. The vast majority of more than 400 described species occurs in the tropics, of which almost half in the Neotropics. With little more than 50 species known from the Holarctic region, the group is relatively poorly represented in temperate regions. This partly explains why the taxonomy of the group has so far received little attention compared to other Syrphidae. This can also be explained by the morphological variation within the Microdontinae, which is arguably larger than in many families of Diptera Cyclorrhapha. Several authors have commented on the group’s paradoxical combination of a wealth of morphological diversity at the species level and a scarceness of group-defining characters (Bezzi 1915, Curran 1941, Shannon 1927). As a result, more than 300 out of approximately 400 valid species names are classified currently in the single genus Microdon Meigen, 1803. This apparent taxonomic indecisiveness seems to result not so much from a lack of morphological variation, but rather from an excess of it.
The classification of taxa, generic as well as specific, within the Microdontinae is the subject of the present paper. All available generic taxa of Microdontinae, as well as many species, are studied and compared in detail. Although phylogenetic relationships are still unclear for many taxa, we prefer to employ an ‘old-fashioned’ method of classification based on detailed comparative morphology over a ‘waste basket’ approach, despite their morphological differences (for more on this see Procedure under Material and Methods). A first phylogenetic analysis of the group is in press (Reemer and Ståhls in press), and in a number of instances the results of that study will be referred to.
When Meigen (1803) introduced the generic name Microdon, there was no intrafamilial classification of the family Syrphidae. The first family group name proposed for Microdon and its allies was Aphritadae Fleming, 1821 (spelled Aphritidae by Fleming 1822), separated from the ‘Syrphadae’ based on the absence of a facial tubercle. The Aphritidae also included Milesia Latreille, 1804 and related genera, which are nowadays included in the Eristalinae. Although the family group name Aphritidae has priority over Microdontinae, the latter name is maintained because Aphritidae has not been used after 1899, whereas Microdontinae has been used by many authors since (ICZN 1999: article 23.9, Sabrosky 1999).
Rondani (1845) first introduced the family group name Microdontinae (spelled as ‘Microdonellae’), based on the dentate scutellum of the type species Microdon mutabilis Linnaeus, 1758. Ever since, this group has been recognized as distinct from other Syrphidae, albeit under different spellings and taxonomic rankings. In early days (Lioy 1864, Brauer 1883, Williston 1886) and the single more recent case of Shatalkin (1975a, b), authors included genera which are nowadays considered to belong to other subfamilies. The placement of the group relative to other Syrphidae, however, has been far from stable. It would exceed the aim of the present paper to repeat here every author’s argumentations for their subsequent classifications over more than one and a half century. Table 1 lists the many different historical taxonomic treatments (spellings and classifications) the group has received.
Chronological overview of spellings, classifications and rankings of the family group names Aphritadae Fleming, 1821 and Microdonellae Rondani, 1845. All known references introducing a novel spelling or classification are included, as well as all known works that explicitly deal with the classification of the group. Works merely using previously suggested classifications are omitted.
| Author | Name / spelling | Ranking and remarks |
|---|---|---|
| Fleming 1821: 55 | Aphritadae | Included Milesia Latreille and related genera. |
| Fleming 1822: 584 | Aphritidae | See Fleming 1821. |
| Rondani 1845: 451 | Microdonellae | One of eight ‘lineas’, equivalent to subfamilies. |
| Rondani 1856: 20, 54 | Microdonina | One of seven lineages, equivalent to subfamilies. |
| Rondani 1857: 206 | Microdoninae | See Rondani 1856. |
| Lioy 1864: 740 | Microdon included in Psariti | One of five subdivisions of Syrphidae, equivalent to subfamilies, including genera Chrysotoxum Meigen, 1803 and Psarus Latreille, 1804. |
| Nowicki 1873: 24 | Microdontina | One of eight subdivisions, equivalent to subfamlies. |
| Brauer 1883: 70 | Microdinae | Equivalent to tribe within subfamily (‘Gruppe’) Chrysotoxinae, including genera Chrysotoxum Meigen, 1803, Pipiza Meigen, Orthonevra Macquart, 1829 among other. |
| Williston 1886: xvi | Microdonini | Tribe within subfamily Syrphinae, including genera Chrysotoxum Meigen, 1803 and Psarus Latreille, 1804. |
| Verrall 1901: 658 | Microdontinae | One of seven subfamilies. |
| Shannon 1921: 67, 123; 1922: 35 | Microdontinae | One of ten subfamilies. |
| Sack 1928-1932: 234 | Microdontinae | One of 14 subfamilies. |
| Hull 1949: 305 | Microdontinae | One of 14 subfamilies, related to Eumerinae and Nausigasterinae. Spheginobaccha included. |
| Goffe 1952: 112 | Microdontina | Subtribe of tribe Volucellini, within subfamily Sphixinae (= Milesiinae of Wirth et al. 1965). |
| Wirth et al. 1965 | Microdontini | Tribe within subfamily Milesiinae |
| Thompson 1969: 75 | Microdontinae | Spheginobaccha excluded. |
| Thompson 1972: 85 | Microdontidae | Family. |
| Shatalkin 1975a, b | Microdontinae | Subfamily. Spheginobaccha included, as well as Alipumilio Shannon, 1927 and Nausigaster Williston, 1884. |
| Speight 1987: 172 | Microdontidae | Family. |
| Ståhls et al. 2003: 449 | Microdontinae | Subfamily. Spheginobaccha included. Alipumilio and Nausigaster excluded. |
| Cheng and Thompson 2008: 21 | Microdontinae | Subfamily. |
| Author | Name / spelling | Ranking and remarks |
|---|---|---|
| Fleming 1821: 55 | Aphritadae | Included Milesia Latreille and related genera. |
| Fleming 1822: 584 | Aphritidae | See Fleming 1821. |
| Rondani 1845: 451 | Microdonellae | One of eight ‘lineas’, equivalent to subfamilies. |
| Rondani 1856: 20, 54 | Microdonina | One of seven lineages, equivalent to subfamilies. |
| Rondani 1857: 206 | Microdoninae | See Rondani 1856. |
| Lioy 1864: 740 | Microdon included in Psariti | One of five subdivisions of Syrphidae, equivalent to subfamilies, including genera Chrysotoxum Meigen, 1803 and Psarus Latreille, 1804. |
| Nowicki 1873: 24 | Microdontina | One of eight subdivisions, equivalent to subfamlies. |
| Brauer 1883: 70 | Microdinae | Equivalent to tribe within subfamily (‘Gruppe’) Chrysotoxinae, including genera Chrysotoxum Meigen, 1803, Pipiza Meigen, Orthonevra Macquart, 1829 among other. |
| Williston 1886: xvi | Microdonini | Tribe within subfamily Syrphinae, including genera Chrysotoxum Meigen, 1803 and Psarus Latreille, 1804. |
| Verrall 1901: 658 | Microdontinae | One of seven subfamilies. |
| Shannon 1921: 67, 123; 1922: 35 | Microdontinae | One of ten subfamilies. |
| Sack 1928-1932: 234 | Microdontinae | One of 14 subfamilies. |
| Hull 1949: 305 | Microdontinae | One of 14 subfamilies, related to Eumerinae and Nausigasterinae. Spheginobaccha included. |
| Goffe 1952: 112 | Microdontina | Subtribe of tribe Volucellini, within subfamily Sphixinae (= Milesiinae of Wirth et al. 1965). |
| Wirth et al. 1965 | Microdontini | Tribe within subfamily Milesiinae |
| Thompson 1969: 75 | Microdontinae | Spheginobaccha excluded. |
| Thompson 1972: 85 | Microdontidae | Family. |
| Shatalkin 1975a, b | Microdontinae | Subfamily. Spheginobaccha included, as well as Alipumilio Shannon, 1927 and Nausigaster Williston, 1884. |
| Speight 1987: 172 | Microdontidae | Family. |
| Ståhls et al. 2003: 449 | Microdontinae | Subfamily. Spheginobaccha included. Alipumilio and Nausigaster excluded. |
| Cheng and Thompson 2008: 21 | Microdontinae | Subfamily. |
The first to regard the Microdontinae as “presumably an old group early differentiated from the family” was Hull (1949). Goffe (1952) extensively reviewed the prior classifications of Syrphidae, including Microdontinae.
He placed the Microdontinae as a subtribe (‘Microdontina’) in the tribe Volucellini, together with the subtribe Volucellina, as part of the subfamily Sphixinae (more or less equivalent to the current Eristalinae). Thompson (1969) did not agree and treated the group again as basal within the Syrphidae. Then Thompson (1972) proposed to raise the group to family level. Shatalkin (1975a, b) did not follow this proposal, basing his argumentation only on the number of male pre-abdominal segments, but he agreed on the basal position of the group as a subfamily within the Syrphidae.
The proposal of Thompson (1972) to treat the Microdontinae as a separate family has not generally been followed. Speight (1987), however, based on his considerations of syrphid morphology, found Microdon to be aberrant from other Syrphidae to such an extent that he chose to follow Thompson’s proposal. In the study of Rotheray and Gilbert (1999), based on characters of immature stages, Microdontinae were placed as follows: (Eristalinae + (Microdontinae + (Syrphinae + Pipizini)). Subsequently, a number of studies recovered the Microdontinae as the sister-group of all other Syrphidae: Skevington and Yeates (2000) (based on molecular data), Ståhls et al. (2003) (based on molecular data combined with larval and adult morphology), and Rotheray and Gilbert (2008) (based on characters of the larval head). The results of Hippa and Ståhls (2005) (based on an extended set of adult morphological characters) differed from those previously mentioned by the placement of (Neoascia Williston, 1886 + Sphegina Meigen, 1822) as sister-group to all other Syrphidae. However, all of these authors treated the Microdontinae as a subfamily, as did Cheng and Thompson (2008). Speight (2010) has, however, continued to use familial rank. Reemer and Ståhls (in press), evaluating previous phylogenetic results as well as their newly generated evidence, see no scientific reason for changing the prevailing ranking of the Microdontinae and for that reason prefer nomenclatural stability.
There have been few previous attempts to generate a tribal classification of Microdontinae. Apart from the names Aphritidae Fleming and Microdontinae Rondani (see previous paragraph), only three family-group names have been proposed: Masarygidae Brèthes, 1908, Ceratophyini Hull, 1949 and Spheginobacchini Thompson, 1972. See Reemer and Ståhls (in press) for discussion on availability of these names. Application of the first two names is at present considered undesirable, as most phylogenetic relationships at suprageneric level are still too uncertain to recognize tribes, due to limited availability of taxa for molecular phylogenetic analysis and the obtained low support values for most of the resolved larger clades (Reemer and Ståhls in press). The tribe Spheginobacchini is the only of these names that continues to be recognized here, as we consider the sister group relationship of this taxon to the remaining Microdontinae well enough established (Ståhls et al. 2003, Reemer and Ståhls in press).
Cheng and Thompson (2008) gave an extensive overview of generic names of Microdontinae, which formed the starting point for the present paper. Since Meigen (1803) introduced the name Microdon, 59 genus-group names applicable to Microdontinae have been introduced (misspellings excluded) (Fig. 1). This number increased most rapidly during the first half of the 20th century. Since then, only nine new genus-group names have been proposed.
Cumulative graph of introduced genus-group names of Microdontinae per decade (as percentage of total number of 59).
Cumulative graph of introduced genus-group names of Microdontinae per decade (as percentage of total number of 59).
The number of previously introduced species-group names in Microdontinae is 514 (including synonyms and unvalid names). The cumulative graph of the number of species names per decade is similar to the one for genus-group names (Fig. 2). A majority of these species names (388) are currently classified into the genus Microdon. Most of the other (sub)genera contain only a few species. The very large genus Microdon thus constitutes one of the greatest taxonomic challenges of Syrphidae. The classification of so many species into one genus was a consequence of pragmaticism, as no comprehensive revisions were available.
Cumulative graph of introduced species-group names of Microdontinae per decade (as percentage of total number of 514).
Cumulative graph of introduced species-group names of Microdontinae per decade (as percentage of total number of 514).
The phylogenetic results of Reemer and Ståhls (in press) are used as a first cue for the generic classification. When the evidence provided by these analyses is not conclusive or considered unconvincing (e.g. because of low support values), morphological characters are evaluated subjectively. Considerable weight is given to the structure of the male genitalia, but in all cases there are also external characters to support the groups. The species classification relies largely on this morphological evaluation.
Generally, a conservative approach is adopted towards changing the rank of taxa. Generic or subgeneric ranks as indicated by Cheng and Thompson (2008) are mostly maintained, unless these are contradicted by the results of the phylogenetic analyses of Reemer and Ståhls (in press). This is mainly relevant in the case of the genus Microdon. The species previously assigned to this genus were resolved as scattered over the phylogenetic trees of Reemer and Ståhls (in press). For some of these groups, genus group names are available, for some there are none. In several cases, genus group names that were previously treated as subgenera are now raised to generic level. In addition, new genus group names needed to be erected for several taxa that were previously included in Microdon. Given the uncertainties in the deeper branches of Microdontinae-phylogeny, these new group names could also have been given subgeneric rank within Microdon. However, this would suggest a close affinity with that genus, despite the fact that this is not indicated by the phylogenetic results. Moreover, we found it useful to split the genus Microdon into smaller natural groups which are more manageable than a genus containing more than 300 species. As this is only done for groups which have a high probability of being monophyletic (as indicated by phylogenetic results of Reemer and Ståhls (in press) or by subjective judgement of supposed synapomorphies), this procedure will facilitate further research on intergeneric phylogenetic relationships.
The following acronyms are used to indicate entomological collections.
AMNH American Museum of Natural History, New York
AMS Australian Museum, Sydney
ANIC Australian National Insect Collection, Canberra
ANSP Academy of Natural Sciences of Pennsylvania, Philadelphia
BMNH British Museum of Natural History, London
CASB Chinese Acadamy of Science, Bejing
CM Carnegie Museum, Pittsburgh
CNC Canadian National Collection, Ottawa
CSCA California State Collection of Athropods, Sacramento
CSCS Central South University of Forestry and Technology, Changsha, Hunan
CU Cornell University, Ithaca
DEI Deutsches Entomologisches Institut, Müncheberg
DZUP Departamento de Zoologia da Universidade Federal do Paraná, Curitiba
HNHM Hungarian Natural History Museum, Budapest
INBIO Instituto Nacional de Biodiversidad, Heredia
MACN Museo Argentino de Ciencias Naturales, Buenos Aires
MCGD Museo Civico di Storia Naturale ‘G. Doria’, Genova
MCSN Museo Civico di Storia Naturale, Milan
MCZ Museum of Comparative Zoology, Harvard
MNHN Muséum National d’Histoire Naturelle, Paris
MRHNB Musée Royal d’Histoire Naturelle de Belgique, Brussels
MRSN Museu Regionale di Scienze Naturali, Turin
MZH Finnish Museum of Natural History, Helsinki
MZLU Museum of Zoology Lund University, Lund
MZM Museum of Zoology, University of Michigan, Ann Arbor
MZUN Museo Zoologico di Università degli Studi, Naples
MZUSP Museu de Zoologia da Universidade de São Paulo, São Paulo
NHRS Naturhistoriska Riksmuseet, Stockholm
NIAS Laboratory of Insect Systematics, National Institute of Agro-Environmental Sciences, Kannondai
NMB Naturhistorisches Museum Basel, Basel
NMSA Natal Museum, Pietermaritzburg
NMW Naturhistorisches Museum Wien, Vienna
NSMT National Science Museum Tokyo, Tokyo
NZCS National Zoological Collection of Surinam, Paramaribo
OHSU Ohio State University, Columbus
OUMNH Oxford University Museum of Natural History, Oxford
RBIN Institut Royal des Sciences Naturelles, Brussels
RMCA Musée Royal de l’Afrique Centrale, Tervuren
RMNH National Museum of Natural History NCB Naturalis, Leiden
QMBA Queensland Museum, Brisbane
QSBG Queen Sirikit Botanical Gardens, Chiang Mai (Thailand)
SAMA South Australian Museum, Adelaide
SAMC South African Museum, Cape Town
SEHU Systematic Entomology Hokkaido University, Sapporo
SEMC Snow Entomological Collections, University of Kansas, Lawrence
SMF Forschungsinstitut und Naturmuseum Senckenberg, Frankfurt
SMNS Staatliches Museum für Naturkunde, Stuttgart
SNSD Senckenberg Naturhistorische Sammlungen Dresden, Dresden
UFPR Universidade Federal dor Paraná, Curitiba
UMSP University of Minnesota, St. Paul
USNM United States National Museum, Smithsonian Institution, Washington D.C.
UTOR Instituto e Museo di Zoologia di Torino, Turin
WSU Washington State University, Pullman
ZFMK Zoologisches Forschungsinstitut und Museum Alexander Koenig, Bonn
ZISP Russian Academy of Sciences, Zoological Institute, St. Petersburg
ZMAN Zoological Museum of Amsterdam - now housed in RMNH, Leiden
ZMHU Zoologisches Museum der Humboldt Universität, Berlin
ZMUC Zoological Museum University of Copenhagen, Copenhagen
ZSI Zoological Survey of India, Calcutta
ZSM Zoologische Staatssammlung, Munich
A few private collections have also been studied. These are referred to in text and appendices by giving the initials and full surname of the owner.
If specimens referred to in the species descriptions in Appendix 1 were used for DNA extraction, this is mentioned by citing the voucher codes on the specimen label (e.g. “DNA voucher G. Ståhls Y0909”). These codes are used by the molecular lab of the Finnish Museum of Natural History (MZH), Helsinki.
Male genitalia were dissected and macerated in an aqueous 10% KOH solution at ambient temperature for 12–24 hours, rinsed in water and stored in glycerol. Drawings of male genitalia were made with the aid of a drawing tube attached to a Wild M20 compound microscope. Photographs of (parts of) specimens were taken through an Olympus SZX12 motorized stereozoom microscope, using Analysis Extended Focal Imaging Software.
Most of the morphological terminology used in this paper is derived from McAlpine (1981), as specifically applied to Syrphidae by Thompson (1999), who also introduced some new terms. Cheng and Thompson (2008) introduced a few more with special relevance to Microdontinae. For some characters used in the present paper, these works do not provide applicable terms. In these cases terminology is based on Hippa and Ståhls (2005) (e.g. antennal fossa, antetergite) and Speight (1987) (e.g. anterolateral callus of tergite 1, anterior sclerite of sternite 2). For the terminology of the male genitalia McAlpine (1981) was used, supplemented with some more recent considerations as summarized by Sinclair (2000) and adopted by Cumming and Wood (2009). This terminology is worked out for Microdontinae in detail in Reemer and Ståhls (in press).
Two keys to genera and generic groups of Microdontinae have been published previously: Hull (1949) and Cheng and Thompson (2008). Characters used in those keys have been considered and some are also used here, but many new characters were necessary to accomodate for new genera and redefined genera. Several taxa are keyed out more than once, either because they are borderline cases or because the key characters are variable between species within these groups. In a few cases the key leads to a species, when this species is an exception among its congeners with regard to the key characters.
A discussion of diagnostic characters of Microdontinae can be found in the Discussion paragraph. A key to distinguish this subfamily from other Syrphidae is also presented there.
| 1 | Postmetacoxal bridge incomplete (metapleura separated from each other) | 97 |
| – | Postmetacoxal bridge complete (metapleura connected posteriad of metacoxa, often only narrowly) | 2 |
| 2 | Vein R4+5 without posterior appendix extending into cell r4+5 (Figs 3, 28, 404) | 74 |
| – | Vein R4+5 with posterior appendix extending into cell r4+5 (Figs 14, 17, 206) | 3 |
| 3 | Postpronotum bare | 67 |
| – | Postpronotum pilose | 4 |
| 4 | Abdomen constricted | 58 |
| – | Abdomen not constricted (oval, parallel-sided or tapering) | 5 |
| 5 | Anepisternum with bare part limited to ventral half of the anepisternum, or entirely pilose | 43 |
| – | Anepisternum extensively bare, with bare part reaching dorsad to above half the height of the anepisternum | 6 |
| 6 | Propleuron (proepimeron) bare | 13 |
| – | Propleuron (proepimeron) pilose | 7 |
| 7 | Postero-apical corner of wing cell r4+5 more or less rectangular or acute, always with small appendix (e.g. Figs 14, 17, 28, 55) | 11 |
| – | Postero-apical corner of wing cell r4+5 widely rounded, sometimes with small appendix (e.g. Figs 177, 206, 210, 289) | 8 |
| 8 | Katepimeron more or less flat (may be a little elevated or with an ill-developed carina, but not convex), sometimes with rows of microtrichia. Abdomen narrow, clearly less than 1.5 times as wide as thorax (Fig. 294) | Peradon: flavofasium-group (in part) |
| – | Katepimeron convex, never with microtrichia. Abdomen wide, about 1.5 times as wide as thorax (Figs 176, 197, 200) | 9 |
| 9 | Apical crossvein M1 with outward angle, usually with a small outward appendix, anteriorly recurrent (Fig. 177) | Microdon (Chymophila) |
| – | Apical crossvein M1 without outward angle (Fig. 206, 210) | 10 |
| 10 | Lateral oral margins not or only slightly produced: anterolateral corners not angular (Fig. 202, 207) | Microdon s.s. |
| – | Lateral oral margins strongly produced: anterolateral corners angular (Fig. 229). | Microdon s.s.: virgo-group |
| 11 | Tergites 3 and 4 not fused, able to articulate independently (Fig. 44) | Ceratophya (in part) |
| – | Tergites 3 and 4 fused, not able to articulate independently, although a suture between the tergites is usually visible (look at lateral margins for best judgement) | 12 |
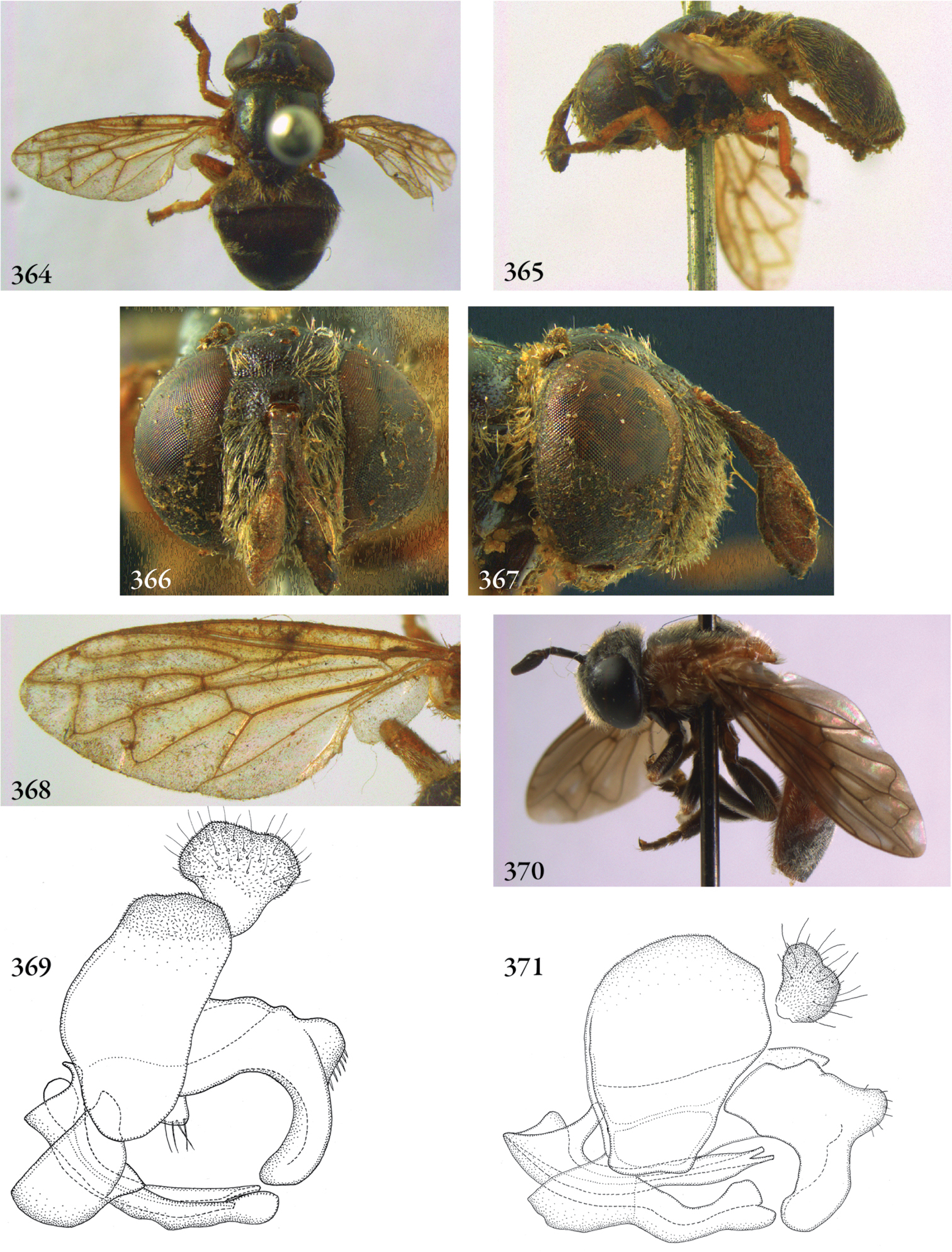
| 12 | Eye bare. Male genitalia: phallus apically furcate (Figs 369, 371, 376) | Serichlamys |
| – | Eye pilose. Male genitalia: phallus unfurcate (Fig. 135) | Laetodon |
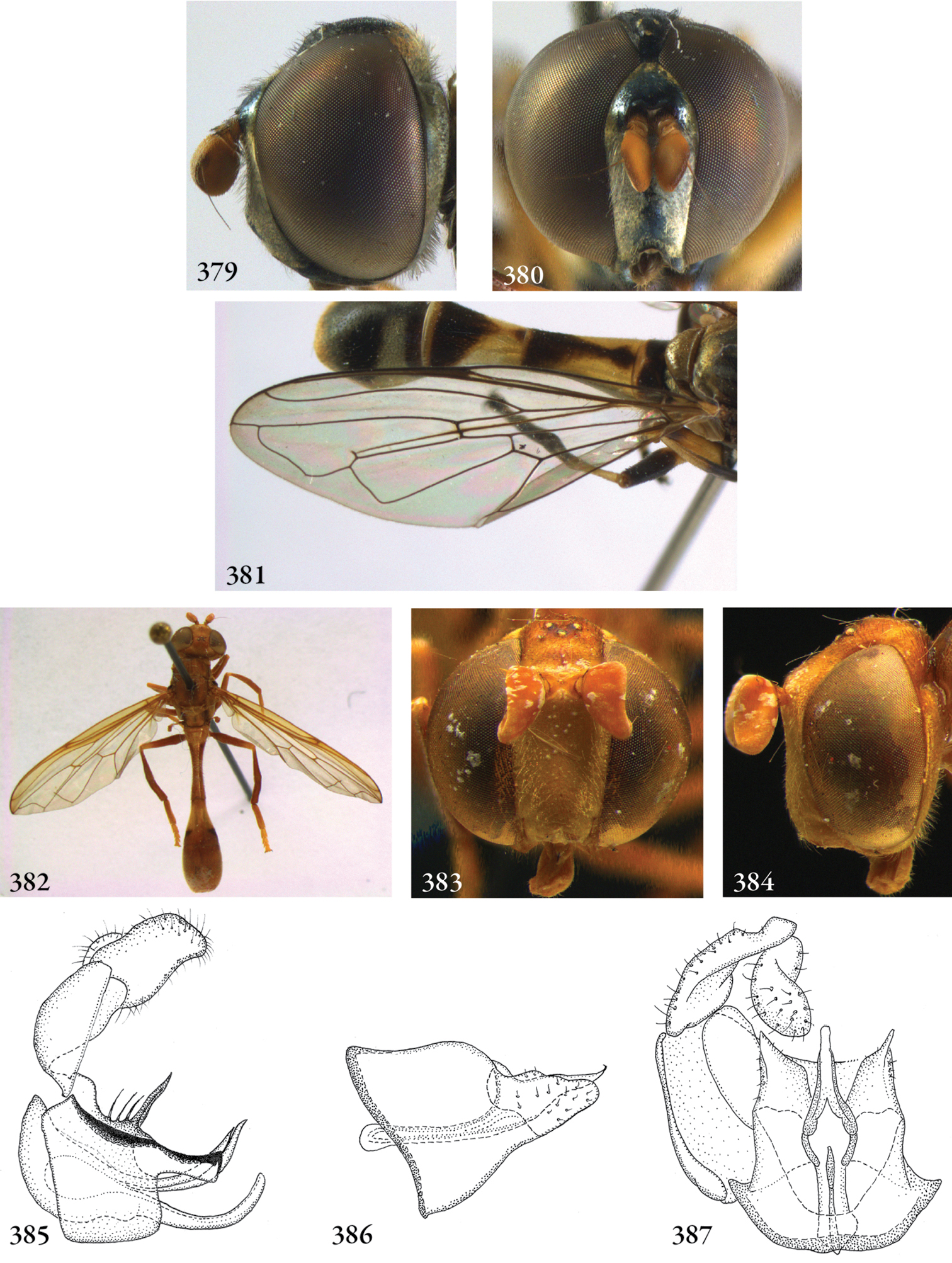
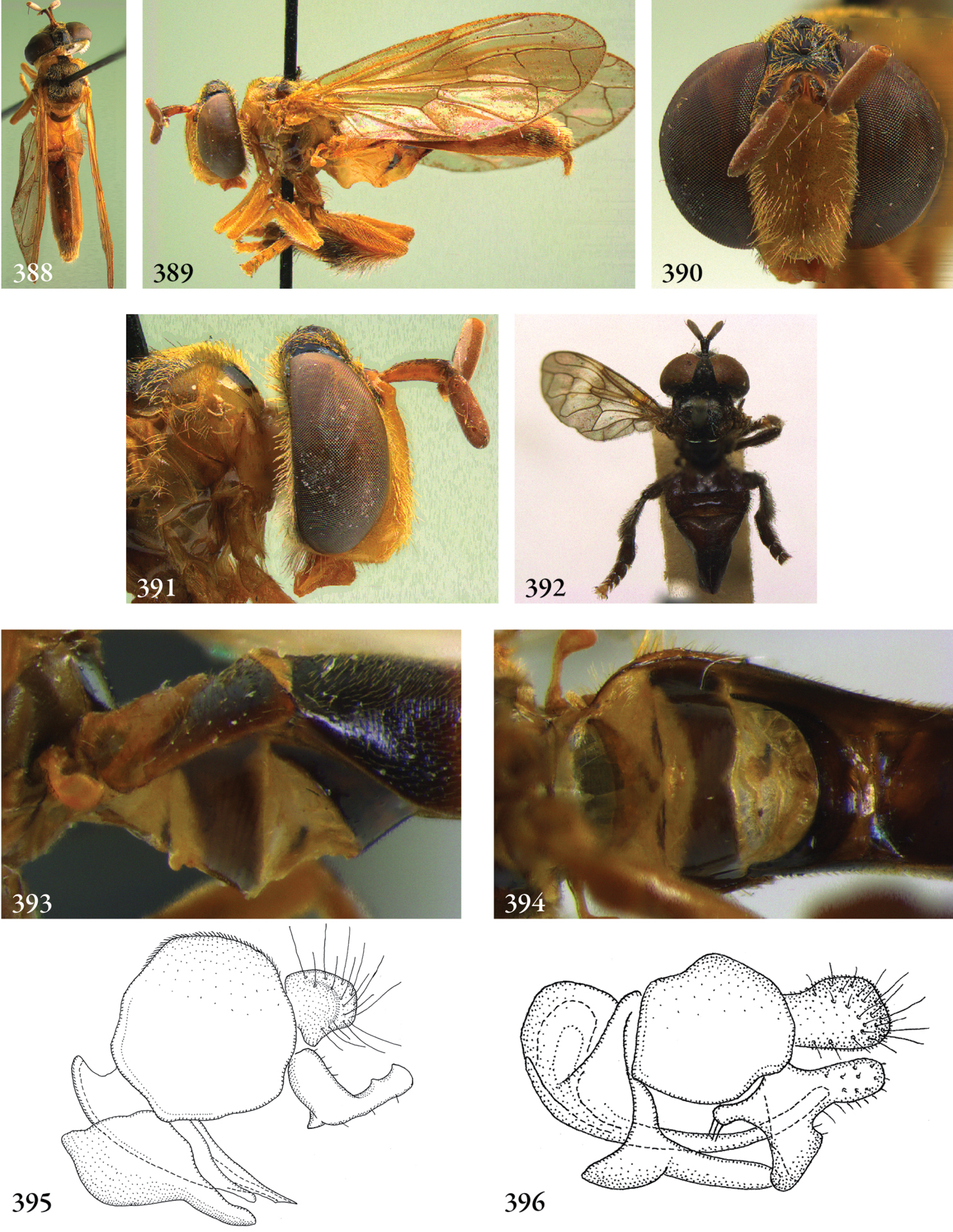
| 13 | Sternites 2 and 3 (often also 1 and 2) separated by unusually wide membraneous part, about as wide as sternite 2 medially or wider (Fig. 393, 394). Antetergite enlarged, longer than tergite 1 medially, almost at level with tergite 1 | Stipomorpha |
| – | Sternites 2 and 3 not separated by unusually wide membraneous part. Antetergite small, shorter than or as long as tergite 1 medially, often not at level with tergite 1 but making a smaller angle | 14 |
| 14 | Postero-apical corner of wing cell r4+5 more or less rectangular or acute (usually with small appendix) (Figs 14, 17, 28, 55) | 29 |
| – | Postero-apical corner of wing cell r4+5 widely rounded (sometimes with small appendix) (Figs 177, 206, 210, 289) | 15 |
| 15 | Basoflagellomere shorter than scape (Fig. 229) | 24 |
| – | Basoflagellomere as long as or longer than scape (Fig. 293) | 16 |
| 16 | Sternite 1 pilose (sometimes only short and sparsely) | 21 |
| – | Sternite 1 bare | 17 |
| 17 | Entire body with metallic green to bluish colouration, densely punctate. Mimics of chrysidid wasps (Hymenoptera: Chrysididae) (Figs 63–67) | Chrysidimyia |
| – | At most thorax with faint metallic hues | 18 |
| 18 | Abdomen constricted basally (Fig. 295) | Peradon: trivittatum-group (in part) |
| – | Abdomen not constricted | 19 |
| 19 | Male with bifurcate basoflagellomere (Fig. 61). Female unknown, possibly with curved or sickle-shaped basoflagellomere. Australian taxon | Cervicorniphora |
| – | Basoflagellomere unfurcate; oval or parallel-sided. Neotropical taxa | 20 |
| 20 | Tergites without fasciae or vittae of golden or silver pile. Basoflagellomere less than twice as long as scape | Peradon: bidens-group |
| – | Tergites usually with fasciae and/or vittae of golden or silver pile. If not, then basoflagellomere more than twice as long as scape | Peradon: flavofascium-group (in part) |
| 21 | Tergite 2 with tubercle halfway on lateral margin (Fig. 421) | Ubristes |
| – | Tergite 2 without tubercle on lateral margin | 22 |
| 22 | Antenna shorter than distance between antennal fossa and anterior oral margin. Basoflagellomere less than twice as long as wide | Microdon rieki (Australia) |
| – | Antenna longer than distance between antennal fossa and anterior oral margin. Basoflagellomere at least four times as long as wide | 23 |
| 23 | Brownish species with long, bee-like pilosity. Scutellum without calcars | Microdon (Myiacerapis) |
| – | Metallic green, sparsely pilose species, reminiscent of chrysidid wasp. Scutellum with calcars | Microdon s.s. (in part: macquartii) |
| 24 | Wings hyaline, at most subtly infuscated | 26 |
| – | Wings with black and yellow colour pattern | 25 |
| 25 | Abdomen without conspicuous fasciae of long pile. Scutellum without calcars. < 20 mm | Microdon s.l.: mirabilis-group |
| – | Abdomen with conspicuous fasciae of long, white pile; apex long, orange pilose. Scutellum with large calcars. >20 mm. Mimics of Eulaema (Hymenoptera: Euglossidae) | Syrphipogon |
| 26 | Vertex convex and shining | Pseudomicrodon (in part: biluminiferus) |
| – | Vertex more or less flat, dull | 27 |
| 27 | Tergites 3 and 4 about equally wide, with lateral margins parallel | Microdon waterhousei Ferguson |
| – | Tergites 3 wider than tergite 4, with lateral margins converging posteriad | 28 |
| 28 | Lateral oral margins strongly produced: anterolateral corners angular (Fig. 229) | Microdon s.s.: virgo-group (in part) |
| – | Lateral oral margins not or only slightly produced: anterolateral corners not angular (Figs 202, 207) | Microdon s.l.: erythros-group |
| 29 | Antenna shorter than distance between antennal fossa and anterior oral margin | 40 |
| – | Antenna as long as or longer than distance between antennal fossa and anterior oral margin | 30 |
| 30 | Scutellum with apical calcars | 34 |
| – | Scutellum without apical calcars, but sometimes sulcate apicomedially or with small patches of microtrichia where calcars could be expected | 31 |
| 31 | Tergites 3 and 4 not fused, able to articulate independently | 33 |
| – | Tergites 3 and 4 fused, not able to articulate independently, although a suture between the tergites is usually visible | 32 |
| 32 | Sternite 1 bare | Menidon falcatus (in part) |
| – | Sternite 1 pilose | Microdon (Dimeraspis) adventitus |
| [The Australian Archimicrodon browni (Thompson) keys here too, but condition of pilosity of sternite 1 unknown.] | ||
| 33 | Male basoflagellomere without long pile. Both sexes: hind tibia in lateral view at least 1.5 times as wide as hind metatarsus | Ceratophya (in part), South America |
| – | Male basoflagellomere with long pile. Both sexes: hind tibia in lateral view about as wide as hind metatarsus | Kryptopyga (in part), Southeast Asia |
| 34 | Occiput dorsally widened (even if only slightly): dorsal eye margin diverging from hind margin of head (Figs 5, 86, 229) | 36 |
| – | Occiput evenly narrow over entire length: dorsal eye margin parallel to hind margin of head (Fig. 191). | 35 |
| 35 | Male: first tarsomere of hind leg dorsally without longitudinal groove; strongly swollen: about twice as wide as apex of hind tibia | Microdon (s.l.) tarsalis |
| – | Male: first tarsomere of hind leg dorsally with wide longitudinal groove; at most 1.5 times as wide as apex of hind tibia | Megodon |
| 36 | Scutellar calcars large and blunt (Fig. 183). Male: first tarsomere of hind leg about twice as wide as apex of hind tibia | Microdon (Dimeraspis) globosus |
| – | Scutellar calcars either absent, very small or well-developed and pointed apically. Male: first tarsomere of hind leg at most 1.5 times as wide as apex of hind tibia | 37 |
| 37 | Vertex convex and shining, bare or sparsely pilose only on posterior half (Figs 70, 71) | Domodon |
| – | Vertex not convex and shining, entirely pilose | 38 |
| 38 | Basoflagellomere oval (Figs 365, 367, 370) | Serichlamys |
| – | Basoflagellomere sickle-shaped (Fig. 154) | 39 |
| 39 | Abdomen largely or entirely yellow (Fig. 151)Menidon falcatus (in part) | |
| – | Abdomen black | Archimicrodon (in part: one undescribed African species) |
| 40 | Anepimeron bare on ventral half. Male with eye margins parallel at level of frons, not approaching | Mermerizon |
| – | Anepimeron entirely pilose. Male with eye margins approaching each other at level of frons. | 41 |
| 41 | Scutellum with large, apically rounded and flattened calcars. | Archimicrodon (Hovamicrodon) |
| – | Scutellum without calcars or with calcars pointed apically | 42 |
| 42 | Male genitalia: surstylus in lateral view without long posterior process (Figs 9, 15) | Archimicrodon s.s. |
| – | Male genitalia: surstylus in lateral view with long posterior process (Figs 19, 22–26) | Archimicrodon s.l. |
| 43 | Basoflagellomere more or less oval or parallel-sided, sometimes with acute apex (Figs 66, 255, 325). | 45 |
| – | Basoflagellomere sickle-shaped or flag-shaped (Figs 252, 425) | 44 |
| 44 | Basoflagellomere sickle-shaped: thickened basally, curved dorsad apically. Arista bare. Eye reduced, so gena, vertex and occiput wide (Fig. 252) | Oligeriops |
| – | Basoflagellomere flag-shaped: strongly widened and laterally flattened (Fig. 425). Arista pilose (pile at least half as long as width of arista). Eyes of normal size | Undescribed genus #1, species AUS-01 |
| 45 | Basoflagellomere shorter than scape | 54 |
| – | Basoflagellomere as long as or longer than scape | 46 |
| 46 | Antenna as long as or longer than distance between antennal fossa and anterior oral margin | 49 |
| – | Antenna shorter than distance between antennal fossa and anterior oral margin | 47 |
| 47 | Tergite 2 with pair of depressed areas (as in Fig. 287); lateral margins of tergite 2 subcircular, widest point clearly before posterior margin | Omegasyrphus |
| – | Tergite 2 without depressed areas; widest point of tergite 2 at posterior margin. | 48 |
| 48 | Wing with conspicuous black markings in apical half (Fig. 230) | Microdon pictipennis |
| – | Wing without conspicuous black markings, only vaguely infuscated along crossveins | Microdon nigromarginalis |
| 49 | Tergites 3 and 4 fused, not able to articulate independently, although a suture between the tergites is usually visible (look at lateral margins for best judgement) | 51 |
| – | Tergites 3 and 4 not fused, able to articulate independently (Fig. 44) | 50 |
| 50 | Dorsal half of occiput slightly widened: maximum width in lateral view less than 1/4 of eye width. Tergite 4 in lateral view approximately perpendicular to tergite 2 (Fig. 43, 44) | Ceratophya |
| – | Dorsal half of occiput strongly widened: maximum width in lateral view about 1/2 of eye width. Tergite 4 in lateral view not perpendicular to tergite 2 | Microdon shirakii |
| 51 | Metallic green species, mimics of chrysidid wasps (Fig. 63, 64) | Chrysidimyia |
| – | Brownish or partly orange species | 52 |
| 52 | Basoflagellomere more than three times as long as scape; with long pilosity in male (Fig. 325) | Ptilobactrum |
| – | Basoflagellomere less than three times as long as scape; bare in male | 53 |
| 53 | Abdomen narrow: more than 1.5 times as long as wide (Figs 163, 167) | Metadon |
| – | Abdomen wide: less than 1.5 times as long as wide (Fig. 181) | Microdon (Dimeraspis) |
| 54 | Abdomen about as long as wide | Microdon (Dimeraspis) abditus |
| – | Abdomen clearly longer than wide | 55 |
| 55 | Metallic green or blue flies, mimics of chrysidid wasps (Fig. 63, 64) | Chrysidimyia |
| – | Not metallic green or blue flies | 56 |
| 56 | Tergite 1 long, with hind margin very rounded; length : width ratio 1:2 or longer (Fig. 81, 83) | Heliodon |
| – | Tergite 1 shorter, with hind margin less rounded; length : width ratio 1:2.5 or shorter | 57 |
| 57 | Tergite 2 with pair of depressed areas (Fig. 287). Abdomen more than 2.5 times as long as wide (Fig. 285). Alula bare | Parocyptamus |
| – | Tergite 2 without depressed areas. Abdomen less than 2.5 times as long as wide (Fig. 163, 167). Alula microtrichose along margins | Metadon |
| 58 | Transverse suture incomplete: not visible medially on mesoscutum | 61 |
| – | Transverse suture complete: extending from one notopleuron to the other | 59 |
| 59 | Katepimeron pilose. Male basoflagellomere with long pile (Fig. 47, 54) | Ceratrichomyia |
| – | Katepimeron bare. Male basoflagellomere without long pile | 60 |
| 60 | Frons laterally without concave area; without sharply defined ridge from lunula to eye margin | Indascia (in part) |
| – | Frons laterally with concave area, covered with dense golden pilosity; ventrally this area is delimited by a sharply defined ridge, which runs from the lunula to the eye margin (Figs 410, 413, 414) | Thompsodon conspicillifrons |
| 61 | Tergites 3 and 4 not fused, able to articulate independently (Fig. 120). Male: sternite 4 not visible in ventral view: completely covered by sternite 3 and lateral margins of tergites (Fig. 123). Male basoflagellomere with long pile (Fig. 122) | Kryptopyga pendulosa |
| – | Tergites 3 and 4 fused, not able to articulate idependently (although a suture between these tergites is usually visible) | 62 |
| 62 | Basoflagellomere longer than scape | 64 |
| – | Basoflagellomere shorter than or as long as scape | 63 |
| 63 | Tergite 2 at most as long as anterior width (Fig. 81) | Heliodon |
| – | Tergite 2 more than twice as long as anterior width (Fig. 347) | Rhopalosyrphus (s.l.) oreokawensis |
| 64 | Vertex convex, shining, sparsely pilose to bare (Fig. 310, 315) | Pseudomicrodon |
| – | Vertex more or less flat, dull and entirely pilose (Fig. 339, 344) | 65 |
| 65 | Tergite 2 with anterior margin about as wide as posterior margin (Fig. 295) | Peradon: trivittatum-group |
| – | Tergite 2 with anterior margin at least 1.5 times as wide as posterior margin (Fig. 332, 337, 342) | 66 |
| 66 | Katepimeron pilose (sometimes only along anterior margin) | Rhopalosyrphus s.s. |
| – | Katepimeron bare | Rhopalosyrphus s.l. |
| 67 | Abdomen oval or elongate, not constricted in dorsal view (Fig. 35, 269, 293, 294) | 69 |
| – | Abdomen constricted in dorsal view (i.e. with narrowest point (in dorsal view) at tergite 2 and widest point at tergite 3 or 4) (Fig. 271, 272, 276) | 68 |
| 68 | Postero-apical corner of wing cell r4+5 widely rounded. Segment 2 longer than thorax (Fig. 59) | Ceriomicrodon petiolatus |
| – | Postero-apical corner of wing cell r4+5 more or less rectangular or acute, with small appendix (Fig. 271). Segment 2 usually shorter than or as long as thorax (Figs 269, 284) (except in one undescribed African taxon) | Paramixogaster (in part) |
| 69 | Basoflagellomere about six times as long as scape (Fig. 36) | Bardistopus |
| – | Basoflagellomere at most four times as long as scape | 70 |
| 70 | Abdomen about as long as wide, with tergite 2 about as long as tergites 3 and 4 together (Fig. 397, 398) | Sulcodon |
| – | Abdomen at least 1.5 times as long as wide, with tergite 2 less than half as long as tergites 3 and 4 together | 71 |
| 71 | Face medially with vitta of transversely wrinkled texture (Fig. 291) | Peradon: flavofascium-group (in part) |
| – | Face medially smooth | 72 |
| 72 | Basoflagellomere longer than scape | Paramixogaster (in part: Paramixogaster acantholepidis, Paramixogaster crematogastri) |
| – | Basoflagellomere shorter than scape | 73 |
| 73 | Tergite 2 twice as wide as long or wider; entirely black | Metadon (in part: Metadon bifasciatus) |
| – | Tergite 2 about 1.5 times as wide as long; with large yellow marking in shape of an inverted “V” | Microdon trigonospilus Bezzi |
| 74 | Vein M anteriorly without small stump extending into cell r4+5 (Fig. 3, 389, 404) | 76 |
| – | Vein M anteriorly with small stump extending into cell r4+5 (Fig. 28, 242, 244) | 75 |
| 75 | Crossvein r-m located between basal 1/4 and 1/3 of cell dm (Fig. 242, 244) | Mixogaster |
| – | Crossvein r-m located within basal 1/7 of cell dm (Fig. 28) | Aristosyrphus (in part: some specimens of Aristosyrphus primus) |
| 76 | Face with median tubercle on dorsal half (Fig. 31) | Aristosyrphus (Eurypterosyrphus) |
| – | Face without median tubercle (Fig. 5) | 77 |
| 77 | Vein M1 more or less straight, not parallel to wing margin, making straight angle with vein R4+5 (Fig. 14, 166, 219) | 79 |
| – | Vein M1 at least in anterior half (sometimes also in posterior half) oblique, more or less parallel to wing margin, making acute angle with vein R4+5 (Fig. 3, 28) | 78 |
| 78 | Abdomen constricted or parallel-sided, not or only slightly wider than thorax (Fig. 27) | Aristosyrphus s.s. |
| – | Abdomen oval, clearly wider than thorax (cf. Fig. 7, 20) | Afromicrodon |
| 79 | Abdomen constricted (i.e. with narrowest point at tergite 2 and widest point at tergite 3 or 4) or elongate and parallel-sided (Figs 103, 262, 284) | 89 |
| – | Abdomen oval (Figs 7, 10, 20, 401) or tapering / triangular (Figs 388, 392) (tergite 2 may be quite narrow anteriorly, but then the abdomen does not get wider beyond posterior margin of tergite 2) | 80 |
| 80 | Sternites 2 and 3 (often also 1 and 2) separated by unusually wide membraneous part, about as wide as sternite 2 medially or wider (Figs 393, 394). Antetergite of tergite 1 enlarged, longer than tergite 1 medially, almost at level with tergite 1 | Stipomorpha |
| – | Sternites 2 and 3 not separated by unusually wide membraneous part. Antetergite small, shorter than or as long as tergite 1 medially, often not at level with tergite 1 but making a smaller angle | 81 |
| 81 | Basoflagellomere shorter than or as long as scape (basoflagellomere never furcate) | 93 |
| – | Basoflagellomere longer than scape (basoflagellomere sometimes furcate in male) | 82 |
| 82 | Antenna at least as long as distance between antennal fossa and anterior oral margin, furcate in male (Figs 39, 77, 138, 144, 356–361) | 86 |
| – | Antenna shorter than distance between antennal fossa and anterior oral margin, never furcate | 83 |
| 83 | Thorax and abdomen black | Archimicrodon s.l. (undescribed taxa from Papua New Guinea) |
| – | Thorax and abdomen yellow and black | 84 |
| 84 | Postpronotum bare | Surimyia |
| – | Postpronotum pilose | 85 |
| 85 | Position of crossvein r-m at same level as bm-cu (Fig. 258) | Paragodon |
| – | Position of crossvein r-m more apical: approximately at basal 1/8 of cell dm | Hypselosyrphus (in part) |
| 86 | Scutellum sulcate apicomedially (cf. Fig. 183) | 88 |
| – | Scutellum not sulcate apicomedially, more or less semicircular | 87 |
| 87 | Antenna inserted dorsally on head: at or above dorsal eye margin. Male basoflagellomere multifurcate (Figs 138, 142–144, 149) | Masarygus |
| – | Antenna inserted below dorsal eye margin. Male basoflagellomere bifurcate (Figs 356–360) | Schizoceratomyia |
| 88 | Katepisternum pilose. Metasternum developed and pilose | Furcantenna |
| – | Katepisternum bare. Metasternum underdeveloped and bare | Carreramyia |
| 89 | Postpronotum pilose | 91 |
| – | Postpronotum bare | 90 |
| 90 | Antenna longer than distance between antennal fossa and anterior oral margin. Basoflagellomere more than 3 times as long as wide (Figs 269–277) | Paramixogaster (in part: Paramixogaster decipiens (de Meijere) and undescribed Australian sp.) |
| – | Antenna shorter than distance between antennal fossa and anterior oral margin. Basoflagellomere less than 2 times as long as wide (Figs 301, 302, 306) | Piruwa |
| 91 | Mesoscutum with transverse suture complete (reaching from one notopleuron to the other) | Indascia |
| – | Mesoscutum with transverse suture not complete (not visible medially) | 92 |
| 92 | Antenna longer than distance between antennal fossa and anterior oral margin. Male basoflagellomere bifurcate (Figs 430, 432) | Undescribed genus #2, species MCR-02 |
| – | Antenna shorter than distance between antennal fossa and anterior oral margin. Male basoflagellomere not furcate (Figs 262–266) | Paramicrodon |
| 93 | Katepimeron pilose | Hypselosyrphus ulopodus |
| – | Katepimeron bare | 94 |
| 94 | Occiput wide, both dorsally and ventrally (Fig. 328) | Rhoga |
| – | Occiput narrow, at least on ventral half (Figs 5, 229, 403) | 95 |
| 95 | Postpronotum bare | Surimyia |
| – | Postpronotum pilose | 96 |
| 96 | Vertex not produced, more or less flat (Figs 3–5) | Afromicrodon |
| – | Vertex produced, more or less convex (Figs 99, 100) | Hypselosyrphus |
| 97 | Abdomen oval or more or less parallel-sided, not constricted (Fig. 330). Occiput without creases (Fig. 328) | Rhoga (in part: maculata, mellea, sepulchrasilva) |
| – | Abdomen elongate and constricted (narrowest point at transition between tergites 2 and 3) (Figs 377, 382). Occiput with distinct creases (Figs 379, 384) | 98 |
| 98 | Proanepisternum without row of long stiff pile. Eye bare | Spheginobaccha (perialla-group) |
| – | Proanepisternum with row of long stiff pile. Eye bare or pilose | 99 |
| 99 | Eye pilose. Alula microtrichose | Spheginobaccha (macropoda-group) |
| – | Eye bare. Alula partially bare | Spheginobaccha (rotundiceps-group) |
| 1 | Postmetacoxal bridge incomplete (metapleura separated from each other) | 97 |
| – | Postmetacoxal bridge complete (metapleura connected posteriad of metacoxa, often only narrowly) | 2 |
| 2 | Vein R4+5 without posterior appendix extending into cell r4+5 (Figs 3, 28, 404) | 74 |
| – | Vein R4+5 with posterior appendix extending into cell r4+5 (Figs 14, 17, 206) | 3 |
| 3 | Postpronotum bare | 67 |
| – | Postpronotum pilose | 4 |
| 4 | Abdomen constricted | 58 |
| – | Abdomen not constricted (oval, parallel-sided or tapering) | 5 |
| 5 | Anepisternum with bare part limited to ventral half of the anepisternum, or entirely pilose | 43 |
| – | Anepisternum extensively bare, with bare part reaching dorsad to above half the height of the anepisternum | 6 |
| 6 | Propleuron (proepimeron) bare | 13 |
| – | Propleuron (proepimeron) pilose | 7 |
| 7 | Postero-apical corner of wing cell r4+5 more or less rectangular or acute, always with small appendix (e.g. Figs 14, 17, 28, 55) | 11 |
| – | Postero-apical corner of wing cell r4+5 widely rounded, sometimes with small appendix (e.g. Figs 177, 206, 210, 289) | 8 |
| 8 | Katepimeron more or less flat (may be a little elevated or with an ill-developed carina, but not convex), sometimes with rows of microtrichia. Abdomen narrow, clearly less than 1.5 times as wide as thorax (Fig. 294) | Peradon: flavofasium-group (in part) |
| – | Katepimeron convex, never with microtrichia. Abdomen wide, about 1.5 times as wide as thorax (Figs 176, 197, 200) | 9 |
| 9 | Apical crossvein M1 with outward angle, usually with a small outward appendix, anteriorly recurrent (Fig. 177) | Microdon (Chymophila) |
| – | Apical crossvein M1 without outward angle (Fig. 206, 210) | 10 |
| 10 | Lateral oral margins not or only slightly produced: anterolateral corners not angular (Fig. 202, 207) | Microdon s.s. |
| – | Lateral oral margins strongly produced: anterolateral corners angular (Fig. 229). | Microdon s.s.: virgo-group |
| 11 | Tergites 3 and 4 not fused, able to articulate independently (Fig. 44) | Ceratophya (in part) |
| – | Tergites 3 and 4 fused, not able to articulate independently, although a suture between the tergites is usually visible (look at lateral margins for best judgement) | 12 |
| 12 | Eye bare. Male genitalia: phallus apically furcate (Figs 369, 371, 376) | Serichlamys |
| – | Eye pilose. Male genitalia: phallus unfurcate (Fig. 135) | Laetodon |
| 13 | Sternites 2 and 3 (often also 1 and 2) separated by unusually wide membraneous part, about as wide as sternite 2 medially or wider (Fig. 393, 394). Antetergite enlarged, longer than tergite 1 medially, almost at level with tergite 1 | Stipomorpha |
| – | Sternites 2 and 3 not separated by unusually wide membraneous part. Antetergite small, shorter than or as long as tergite 1 medially, often not at level with tergite 1 but making a smaller angle | 14 |
| 14 | Postero-apical corner of wing cell r4+5 more or less rectangular or acute (usually with small appendix) (Figs 14, 17, 28, 55) | 29 |
| – | Postero-apical corner of wing cell r4+5 widely rounded (sometimes with small appendix) (Figs 177, 206, 210, 289) | 15 |
| 15 | Basoflagellomere shorter than scape (Fig. 229) | 24 |
| – | Basoflagellomere as long as or longer than scape (Fig. 293) | 16 |
| 16 | Sternite 1 pilose (sometimes only short and sparsely) | 21 |
| – | Sternite 1 bare | 17 |
| 17 | Entire body with metallic green to bluish colouration, densely punctate. Mimics of chrysidid wasps (Hymenoptera: Chrysididae) (Figs 63–67) | Chrysidimyia |
| – | At most thorax with faint metallic hues | 18 |
| 18 | Abdomen constricted basally (Fig. 295) | Peradon: trivittatum-group (in part) |
| – | Abdomen not constricted | 19 |
| 19 | Male with bifurcate basoflagellomere (Fig. 61). Female unknown, possibly with curved or sickle-shaped basoflagellomere. Australian taxon | Cervicorniphora |
| – | Basoflagellomere unfurcate; oval or parallel-sided. Neotropical taxa | 20 |
| 20 | Tergites without fasciae or vittae of golden or silver pile. Basoflagellomere less than twice as long as scape | Peradon: bidens-group |
| – | Tergites usually with fasciae and/or vittae of golden or silver pile. If not, then basoflagellomere more than twice as long as scape | Peradon: flavofascium-group (in part) |
| 21 | Tergite 2 with tubercle halfway on lateral margin (Fig. 421) | Ubristes |
| – | Tergite 2 without tubercle on lateral margin | 22 |
| 22 | Antenna shorter than distance between antennal fossa and anterior oral margin. Basoflagellomere less than twice as long as wide | Microdon rieki (Australia) |
| – | Antenna longer than distance between antennal fossa and anterior oral margin. Basoflagellomere at least four times as long as wide | 23 |
| 23 | Brownish species with long, bee-like pilosity. Scutellum without calcars | Microdon (Myiacerapis) |
| – | Metallic green, sparsely pilose species, reminiscent of chrysidid wasp. Scutellum with calcars | Microdon s.s. (in part: macquartii) |
| 24 | Wings hyaline, at most subtly infuscated | 26 |
| – | Wings with black and yellow colour pattern | 25 |
| 25 | Abdomen without conspicuous fasciae of long pile. Scutellum without calcars. < 20 mm | Microdon s.l.: mirabilis-group |
| – | Abdomen with conspicuous fasciae of long, white pile; apex long, orange pilose. Scutellum with large calcars. >20 mm. Mimics of Eulaema (Hymenoptera: Euglossidae) | Syrphipogon |
| 26 | Vertex convex and shining | Pseudomicrodon (in part: biluminiferus) |
| – | Vertex more or less flat, dull | 27 |
| 27 | Tergites 3 and 4 about equally wide, with lateral margins parallel | Microdon waterhousei Ferguson |
| – | Tergites 3 wider than tergite 4, with lateral margins converging posteriad | 28 |
| 28 | Lateral oral margins strongly produced: anterolateral corners angular (Fig. 229) | Microdon s.s.: virgo-group (in part) |
| – | Lateral oral margins not or only slightly produced: anterolateral corners not angular (Figs 202, 207) | Microdon s.l.: erythros-group |
| 29 | Antenna shorter than distance between antennal fossa and anterior oral margin | 40 |
| – | Antenna as long as or longer than distance between antennal fossa and anterior oral margin | 30 |
| 30 | Scutellum with apical calcars | 34 |
| – | Scutellum without apical calcars, but sometimes sulcate apicomedially or with small patches of microtrichia where calcars could be expected | 31 |
| 31 | Tergites 3 and 4 not fused, able to articulate independently | 33 |
| – | Tergites 3 and 4 fused, not able to articulate independently, although a suture between the tergites is usually visible | 32 |
| 32 | Sternite 1 bare | Menidon falcatus (in part) |
| – | Sternite 1 pilose | Microdon (Dimeraspis) adventitus |
| [The Australian Archimicrodon browni (Thompson) keys here too, but condition of pilosity of sternite 1 unknown.] | ||
| 33 | Male basoflagellomere without long pile. Both sexes: hind tibia in lateral view at least 1.5 times as wide as hind metatarsus | Ceratophya (in part), South America |
| – | Male basoflagellomere with long pile. Both sexes: hind tibia in lateral view about as wide as hind metatarsus | Kryptopyga (in part), Southeast Asia |
| 34 | Occiput dorsally widened (even if only slightly): dorsal eye margin diverging from hind margin of head (Figs 5, 86, 229) | 36 |
| – | Occiput evenly narrow over entire length: dorsal eye margin parallel to hind margin of head (Fig. 191). | 35 |
| 35 | Male: first tarsomere of hind leg dorsally without longitudinal groove; strongly swollen: about twice as wide as apex of hind tibia | Microdon (s.l.) tarsalis |
| – | Male: first tarsomere of hind leg dorsally with wide longitudinal groove; at most 1.5 times as wide as apex of hind tibia | Megodon |
| 36 | Scutellar calcars large and blunt (Fig. 183). Male: first tarsomere of hind leg about twice as wide as apex of hind tibia | Microdon (Dimeraspis) globosus |
| – | Scutellar calcars either absent, very small or well-developed and pointed apically. Male: first tarsomere of hind leg at most 1.5 times as wide as apex of hind tibia | 37 |
| 37 | Vertex convex and shining, bare or sparsely pilose only on posterior half (Figs 70, 71) | Domodon |
| – | Vertex not convex and shining, entirely pilose | 38 |
| 38 | Basoflagellomere oval (Figs 365, 367, 370) | Serichlamys |
| – | Basoflagellomere sickle-shaped (Fig. 154) | 39 |
| 39 | Abdomen largely or entirely yellow (Fig. 151)Menidon falcatus (in part) | |
| – | Abdomen black | Archimicrodon (in part: one undescribed African species) |
| 40 | Anepimeron bare on ventral half. Male with eye margins parallel at level of frons, not approaching | Mermerizon |
| – | Anepimeron entirely pilose. Male with eye margins approaching each other at level of frons. | 41 |
| 41 | Scutellum with large, apically rounded and flattened calcars. | Archimicrodon (Hovamicrodon) |
| – | Scutellum without calcars or with calcars pointed apically | 42 |
| 42 | Male genitalia: surstylus in lateral view without long posterior process (Figs 9, 15) | Archimicrodon s.s. |
| – | Male genitalia: surstylus in lateral view with long posterior process (Figs 19, 22–26) | Archimicrodon s.l. |
| 43 | Basoflagellomere more or less oval or parallel-sided, sometimes with acute apex (Figs 66, 255, 325). | 45 |
| – | Basoflagellomere sickle-shaped or flag-shaped (Figs 252, 425) | 44 |
| 44 | Basoflagellomere sickle-shaped: thickened basally, curved dorsad apically. Arista bare. Eye reduced, so gena, vertex and occiput wide (Fig. 252) | Oligeriops |
| – | Basoflagellomere flag-shaped: strongly widened and laterally flattened (Fig. 425). Arista pilose (pile at least half as long as width of arista). Eyes of normal size | Undescribed genus #1, species AUS-01 |
| 45 | Basoflagellomere shorter than scape | 54 |
| – | Basoflagellomere as long as or longer than scape | 46 |
| 46 | Antenna as long as or longer than distance between antennal fossa and anterior oral margin | 49 |
| – | Antenna shorter than distance between antennal fossa and anterior oral margin | 47 |
| 47 | Tergite 2 with pair of depressed areas (as in Fig. 287); lateral margins of tergite 2 subcircular, widest point clearly before posterior margin | Omegasyrphus |
| – | Tergite 2 without depressed areas; widest point of tergite 2 at posterior margin. | 48 |
| 48 | Wing with conspicuous black markings in apical half (Fig. 230) | Microdon pictipennis |
| – | Wing without conspicuous black markings, only vaguely infuscated along crossveins | Microdon nigromarginalis |
| 49 | Tergites 3 and 4 fused, not able to articulate independently, although a suture between the tergites is usually visible (look at lateral margins for best judgement) | 51 |
| – | Tergites 3 and 4 not fused, able to articulate independently (Fig. 44) | 50 |
| 50 | Dorsal half of occiput slightly widened: maximum width in lateral view less than 1/4 of eye width. Tergite 4 in lateral view approximately perpendicular to tergite 2 (Fig. 43, 44) | Ceratophya |
| – | Dorsal half of occiput strongly widened: maximum width in lateral view about 1/2 of eye width. Tergite 4 in lateral view not perpendicular to tergite 2 | Microdon shirakii |
| 51 | Metallic green species, mimics of chrysidid wasps (Fig. 63, 64) | Chrysidimyia |
| – | Brownish or partly orange species | 52 |
| 52 | Basoflagellomere more than three times as long as scape; with long pilosity in male (Fig. 325) | Ptilobactrum |
| – | Basoflagellomere less than three times as long as scape; bare in male | 53 |
| 53 | Abdomen narrow: more than 1.5 times as long as wide (Figs 163, 167) | Metadon |
| – | Abdomen wide: less than 1.5 times as long as wide (Fig. 181) | Microdon (Dimeraspis) |
| 54 | Abdomen about as long as wide | Microdon (Dimeraspis) abditus |
| – | Abdomen clearly longer than wide | 55 |
| 55 | Metallic green or blue flies, mimics of chrysidid wasps (Fig. 63, 64) | Chrysidimyia |
| – | Not metallic green or blue flies | 56 |
| 56 | Tergite 1 long, with hind margin very rounded; length : width ratio 1:2 or longer (Fig. 81, 83) | Heliodon |
| – | Tergite 1 shorter, with hind margin less rounded; length : width ratio 1:2.5 or shorter | 57 |
| 57 | Tergite 2 with pair of depressed areas (Fig. 287). Abdomen more than 2.5 times as long as wide (Fig. 285). Alula bare | Parocyptamus |
| – | Tergite 2 without depressed areas. Abdomen less than 2.5 times as long as wide (Fig. 163, 167). Alula microtrichose along margins | Metadon |
| 58 | Transverse suture incomplete: not visible medially on mesoscutum | 61 |
| – | Transverse suture complete: extending from one notopleuron to the other | 59 |
| 59 | Katepimeron pilose. Male basoflagellomere with long pile (Fig. 47, 54) | Ceratrichomyia |
| – | Katepimeron bare. Male basoflagellomere without long pile | 60 |
| 60 | Frons laterally without concave area; without sharply defined ridge from lunula to eye margin | Indascia (in part) |
| – | Frons laterally with concave area, covered with dense golden pilosity; ventrally this area is delimited by a sharply defined ridge, which runs from the lunula to the eye margin (Figs 410, 413, 414) | Thompsodon conspicillifrons |
| 61 | Tergites 3 and 4 not fused, able to articulate independently (Fig. 120). Male: sternite 4 not visible in ventral view: completely covered by sternite 3 and lateral margins of tergites (Fig. 123). Male basoflagellomere with long pile (Fig. 122) | Kryptopyga pendulosa |
| – | Tergites 3 and 4 fused, not able to articulate idependently (although a suture between these tergites is usually visible) | 62 |
| 62 | Basoflagellomere longer than scape | 64 |
| – | Basoflagellomere shorter than or as long as scape | 63 |
| 63 | Tergite 2 at most as long as anterior width (Fig. 81) | Heliodon |
| – | Tergite 2 more than twice as long as anterior width (Fig. 347) | Rhopalosyrphus (s.l.) oreokawensis |
| 64 | Vertex convex, shining, sparsely pilose to bare (Fig. 310, 315) | Pseudomicrodon |
| – | Vertex more or less flat, dull and entirely pilose (Fig. 339, 344) | 65 |
| 65 | Tergite 2 with anterior margin about as wide as posterior margin (Fig. 295) | Peradon: trivittatum-group |
| – | Tergite 2 with anterior margin at least 1.5 times as wide as posterior margin (Fig. 332, 337, 342) | 66 |
| 66 | Katepimeron pilose (sometimes only along anterior margin) | Rhopalosyrphus s.s. |
| – | Katepimeron bare | Rhopalosyrphus s.l. |
| 67 | Abdomen oval or elongate, not constricted in dorsal view (Fig. 35, 269, 293, 294) | 69 |
| – | Abdomen constricted in dorsal view (i.e. with narrowest point (in dorsal view) at tergite 2 and widest point at tergite 3 or 4) (Fig. 271, 272, 276) | 68 |
| 68 | Postero-apical corner of wing cell r4+5 widely rounded. Segment 2 longer than thorax (Fig. 59) | Ceriomicrodon petiolatus |
| – | Postero-apical corner of wing cell r4+5 more or less rectangular or acute, with small appendix (Fig. 271). Segment 2 usually shorter than or as long as thorax (Figs 269, 284) (except in one undescribed African taxon) | Paramixogaster (in part) |
| 69 | Basoflagellomere about six times as long as scape (Fig. 36) | Bardistopus |
| – | Basoflagellomere at most four times as long as scape | 70 |
| 70 | Abdomen about as long as wide, with tergite 2 about as long as tergites 3 and 4 together (Fig. 397, 398) | Sulcodon |
| – | Abdomen at least 1.5 times as long as wide, with tergite 2 less than half as long as tergites 3 and 4 together | 71 |
| 71 | Face medially with vitta of transversely wrinkled texture (Fig. 291) | Peradon: flavofascium-group (in part) |
| – | Face medially smooth | 72 |
| 72 | Basoflagellomere longer than scape | Paramixogaster (in part: Paramixogaster acantholepidis, Paramixogaster crematogastri) |
| – | Basoflagellomere shorter than scape | 73 |
| 73 | Tergite 2 twice as wide as long or wider; entirely black | Metadon (in part: Metadon bifasciatus) |
| – | Tergite 2 about 1.5 times as wide as long; with large yellow marking in shape of an inverted “V” | Microdon trigonospilus Bezzi |
| 74 | Vein M anteriorly without small stump extending into cell r4+5 (Fig. 3, 389, 404) | 76 |
| – | Vein M anteriorly with small stump extending into cell r4+5 (Fig. 28, 242, 244) | 75 |
| 75 | Crossvein r-m located between basal 1/4 and 1/3 of cell dm (Fig. 242, 244) | Mixogaster |
| – | Crossvein r-m located within basal 1/7 of cell dm (Fig. 28) | Aristosyrphus (in part: some specimens of Aristosyrphus primus) |
| 76 | Face with median tubercle on dorsal half (Fig. 31) | Aristosyrphus (Eurypterosyrphus) |
| – | Face without median tubercle (Fig. 5) | 77 |
| 77 | Vein M1 more or less straight, not parallel to wing margin, making straight angle with vein R4+5 (Fig. 14, 166, 219) | 79 |
| – | Vein M1 at least in anterior half (sometimes also in posterior half) oblique, more or less parallel to wing margin, making acute angle with vein R4+5 (Fig. 3, 28) | 78 |
| 78 | Abdomen constricted or parallel-sided, not or only slightly wider than thorax (Fig. 27) | Aristosyrphus s.s. |
| – | Abdomen oval, clearly wider than thorax (cf. Fig. 7, 20) | Afromicrodon |
| 79 | Abdomen constricted (i.e. with narrowest point at tergite 2 and widest point at tergite 3 or 4) or elongate and parallel-sided (Figs 103, 262, 284) | 89 |
| – | Abdomen oval (Figs 7, 10, 20, 401) or tapering / triangular (Figs 388, 392) (tergite 2 may be quite narrow anteriorly, but then the abdomen does not get wider beyond posterior margin of tergite 2) | 80 |
| 80 | Sternites 2 and 3 (often also 1 and 2) separated by unusually wide membraneous part, about as wide as sternite 2 medially or wider (Figs 393, 394). Antetergite of tergite 1 enlarged, longer than tergite 1 medially, almost at level with tergite 1 | Stipomorpha |
| – | Sternites 2 and 3 not separated by unusually wide membraneous part. Antetergite small, shorter than or as long as tergite 1 medially, often not at level with tergite 1 but making a smaller angle | 81 |
| 81 | Basoflagellomere shorter than or as long as scape (basoflagellomere never furcate) | 93 |
| – | Basoflagellomere longer than scape (basoflagellomere sometimes furcate in male) | 82 |
| 82 | Antenna at least as long as distance between antennal fossa and anterior oral margin, furcate in male (Figs 39, 77, 138, 144, 356–361) | 86 |
| – | Antenna shorter than distance between antennal fossa and anterior oral margin, never furcate | 83 |
| 83 | Thorax and abdomen black | Archimicrodon s.l. (undescribed taxa from Papua New Guinea) |
| – | Thorax and abdomen yellow and black | 84 |
| 84 | Postpronotum bare | Surimyia |
| – | Postpronotum pilose | 85 |
| 85 | Position of crossvein r-m at same level as bm-cu (Fig. 258) | Paragodon |
| – | Position of crossvein r-m more apical: approximately at basal 1/8 of cell dm | Hypselosyrphus (in part) |
| 86 | Scutellum sulcate apicomedially (cf. Fig. 183) | 88 |
| – | Scutellum not sulcate apicomedially, more or less semicircular | 87 |
| 87 | Antenna inserted dorsally on head: at or above dorsal eye margin. Male basoflagellomere multifurcate (Figs 138, 142–144, 149) | Masarygus |
| – | Antenna inserted below dorsal eye margin. Male basoflagellomere bifurcate (Figs 356–360) | Schizoceratomyia |
| 88 | Katepisternum pilose. Metasternum developed and pilose | Furcantenna |
| – | Katepisternum bare. Metasternum underdeveloped and bare | Carreramyia |
| 89 | Postpronotum pilose | 91 |
| – | Postpronotum bare | 90 |
| 90 | Antenna longer than distance between antennal fossa and anterior oral margin. Basoflagellomere more than 3 times as long as wide (Figs 269–277) | Paramixogaster (in part: Paramixogaster decipiens (de Meijere) and undescribed Australian sp.) |
| – | Antenna shorter than distance between antennal fossa and anterior oral margin. Basoflagellomere less than 2 times as long as wide (Figs 301, 302, 306) | Piruwa |
| 91 | Mesoscutum with transverse suture complete (reaching from one notopleuron to the other) | Indascia |
| – | Mesoscutum with transverse suture not complete (not visible medially) | 92 |
| 92 | Antenna longer than distance between antennal fossa and anterior oral margin. Male basoflagellomere bifurcate (Figs 430, 432) | Undescribed genus #2, species MCR-02 |
| – | Antenna shorter than distance between antennal fossa and anterior oral margin. Male basoflagellomere not furcate (Figs 262–266) | Paramicrodon |
| 93 | Katepimeron pilose | Hypselosyrphus ulopodus |
| – | Katepimeron bare | 94 |
| 94 | Occiput wide, both dorsally and ventrally (Fig. 328) | Rhoga |
| – | Occiput narrow, at least on ventral half (Figs 5, 229, 403) | 95 |
| 95 | Postpronotum bare | Surimyia |
| – | Postpronotum pilose | 96 |
| 96 | Vertex not produced, more or less flat (Figs 3–5) | Afromicrodon |
| – | Vertex produced, more or less convex (Figs 99, 100) | Hypselosyrphus |
| 97 | Abdomen oval or more or less parallel-sided, not constricted (Fig. 330). Occiput without creases (Fig. 328) | Rhoga (in part: maculata, mellea, sepulchrasilva) |
| – | Abdomen elongate and constricted (narrowest point at transition between tergites 2 and 3) (Figs 377, 382). Occiput with distinct creases (Figs 379, 384) | 98 |
| 98 | Proanepisternum without row of long stiff pile. Eye bare | Spheginobaccha (perialla-group) |
| – | Proanepisternum with row of long stiff pile. Eye bare or pilose | 99 |
| 99 | Eye pilose. Alula microtrichose | Spheginobaccha (macropoda-group) |
| – | Eye bare. Alula partially bare | Spheginobaccha (rotundiceps-group) |
The genera accounts are presented in alphabetic order. Accounts are only given for taxa considered as valid genera or subgenera. Synonyms and misspelled names can be found under the valid genera to which they belong. Each group account starts with information on the original description and the type species. This is followed by the following components.
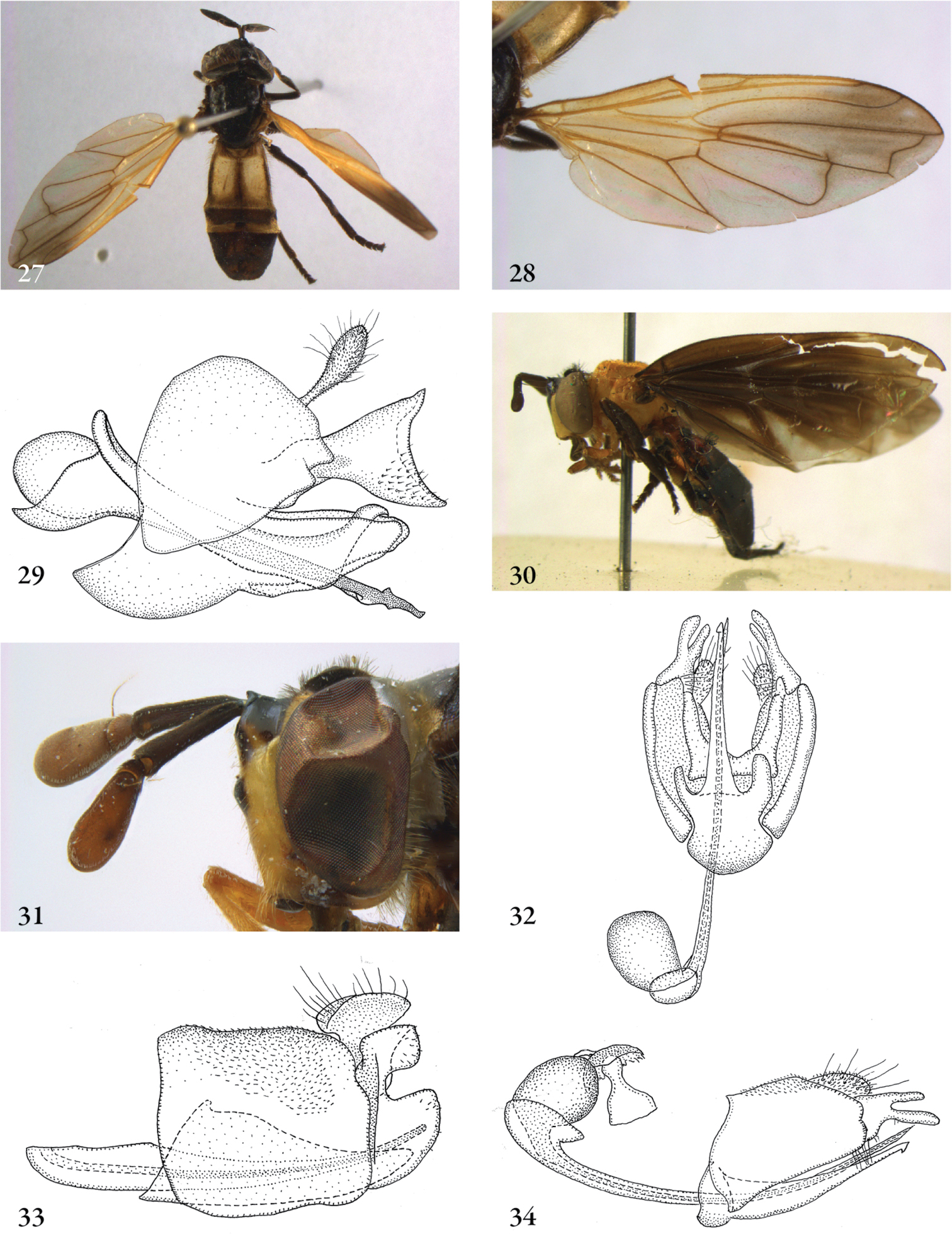
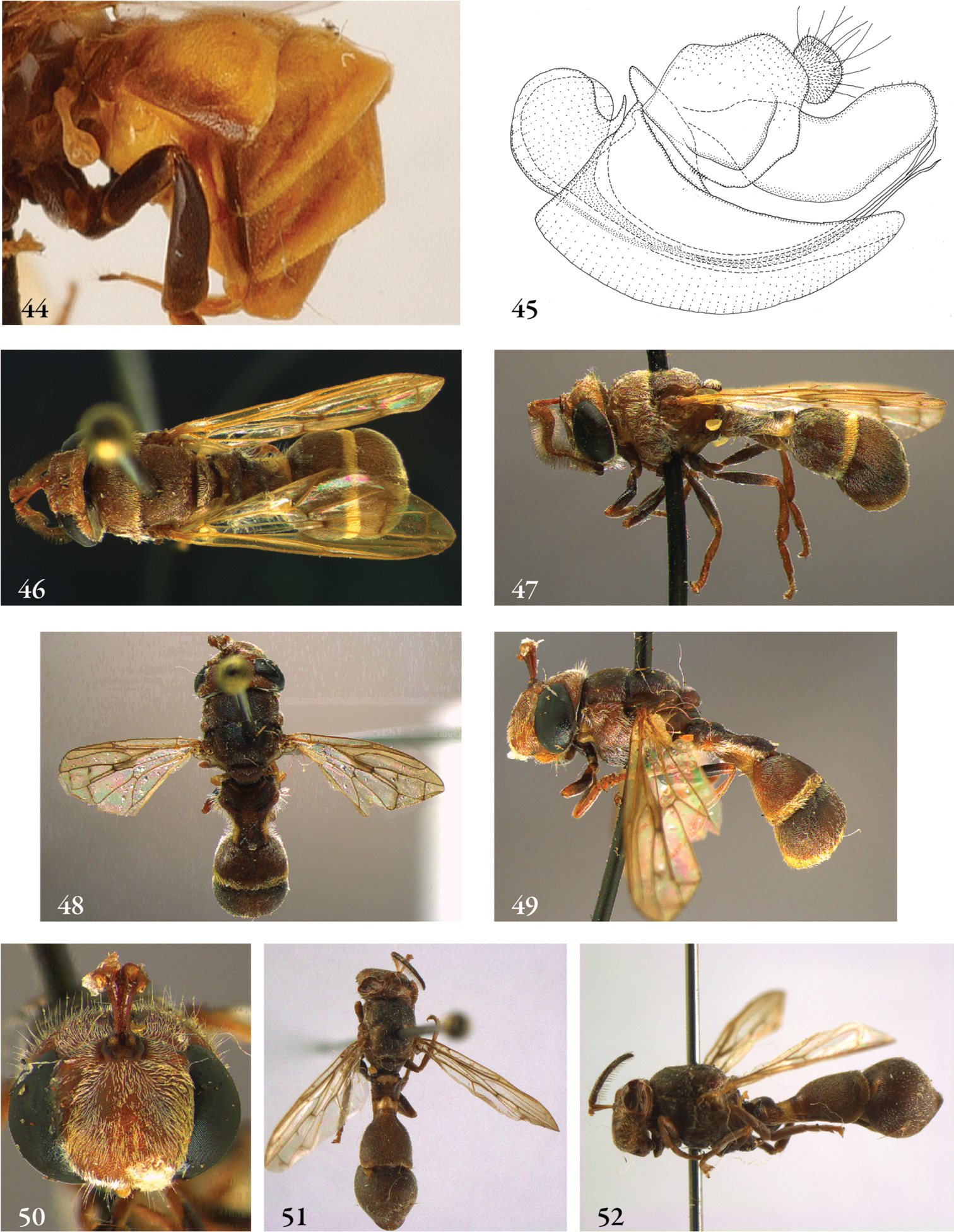
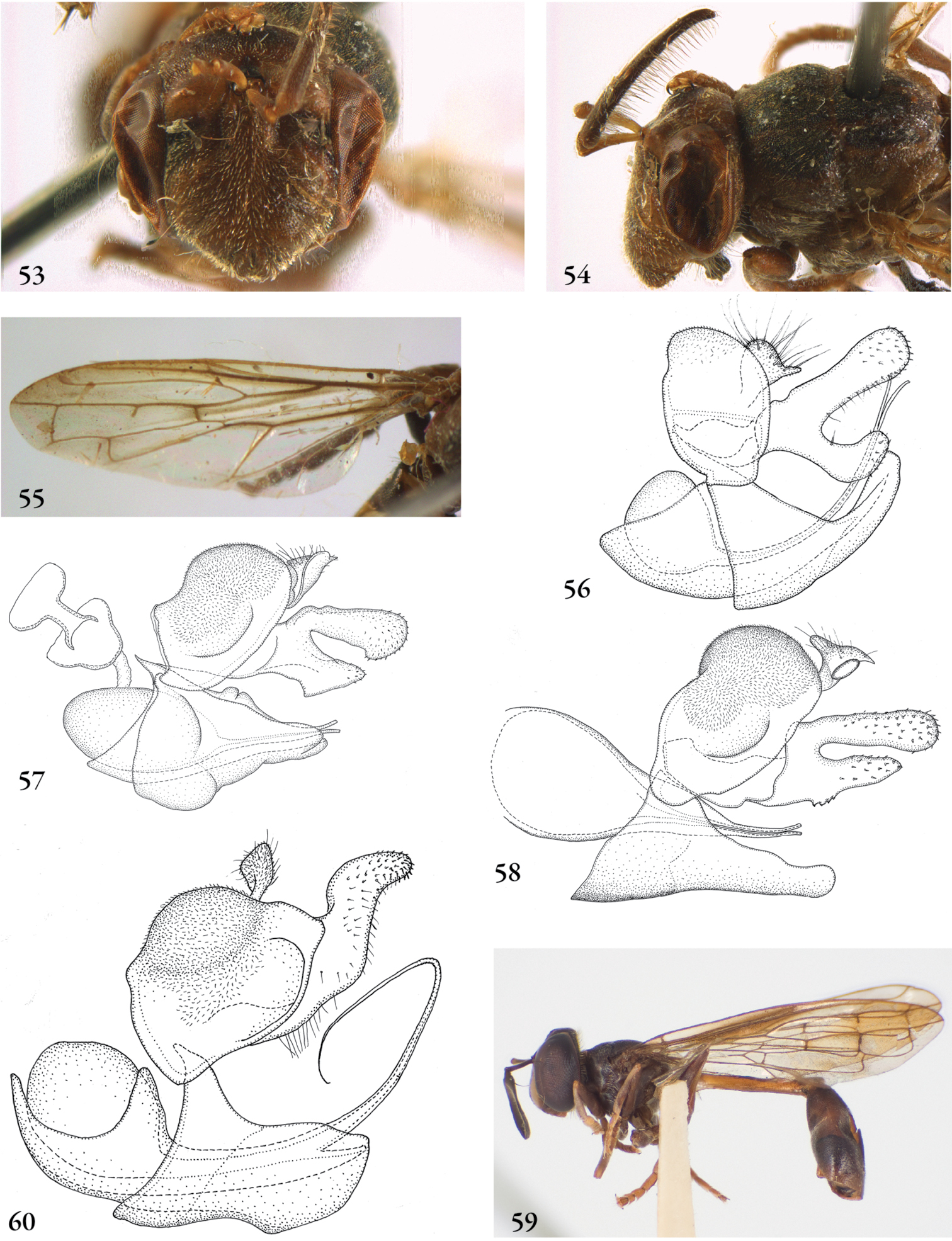
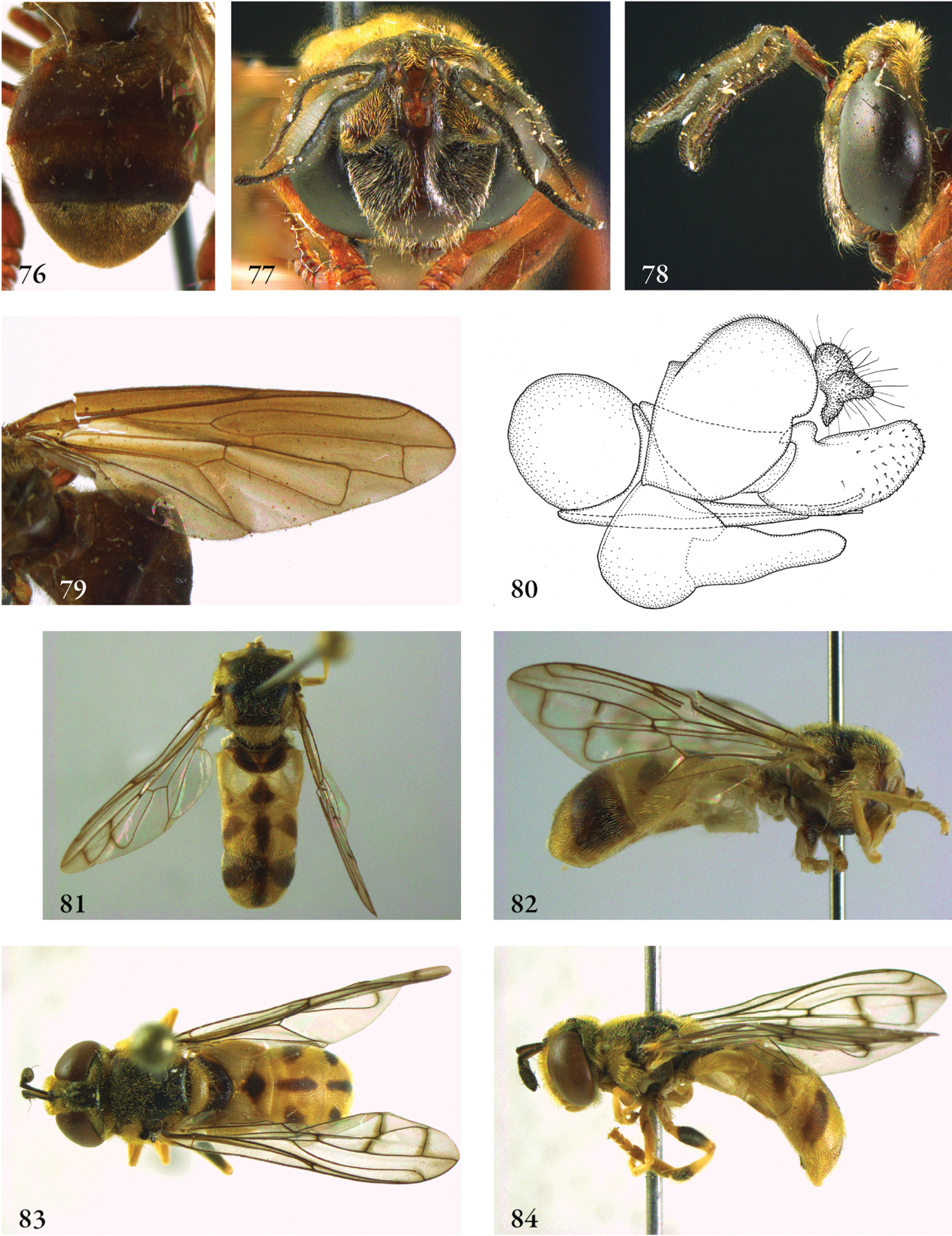
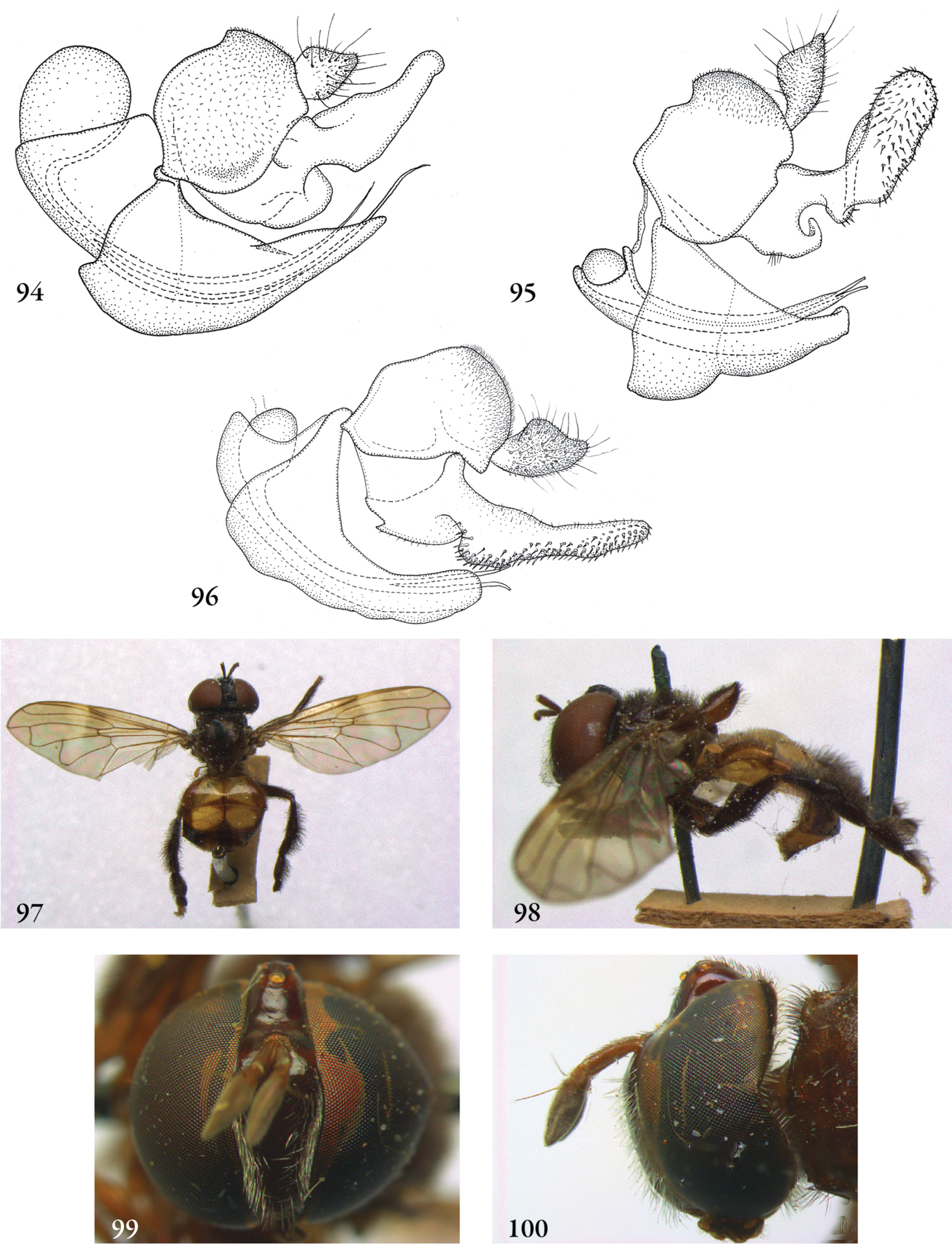
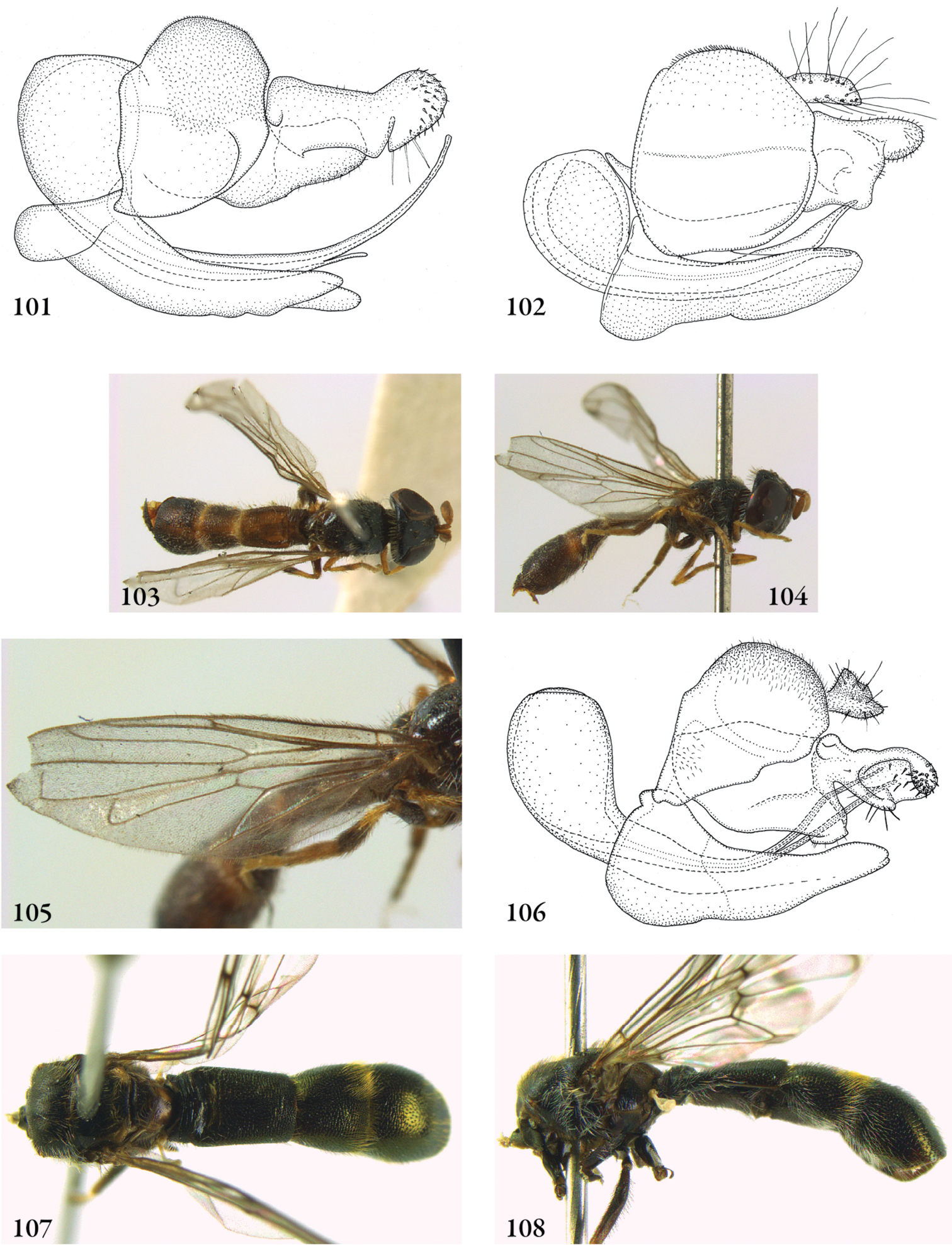
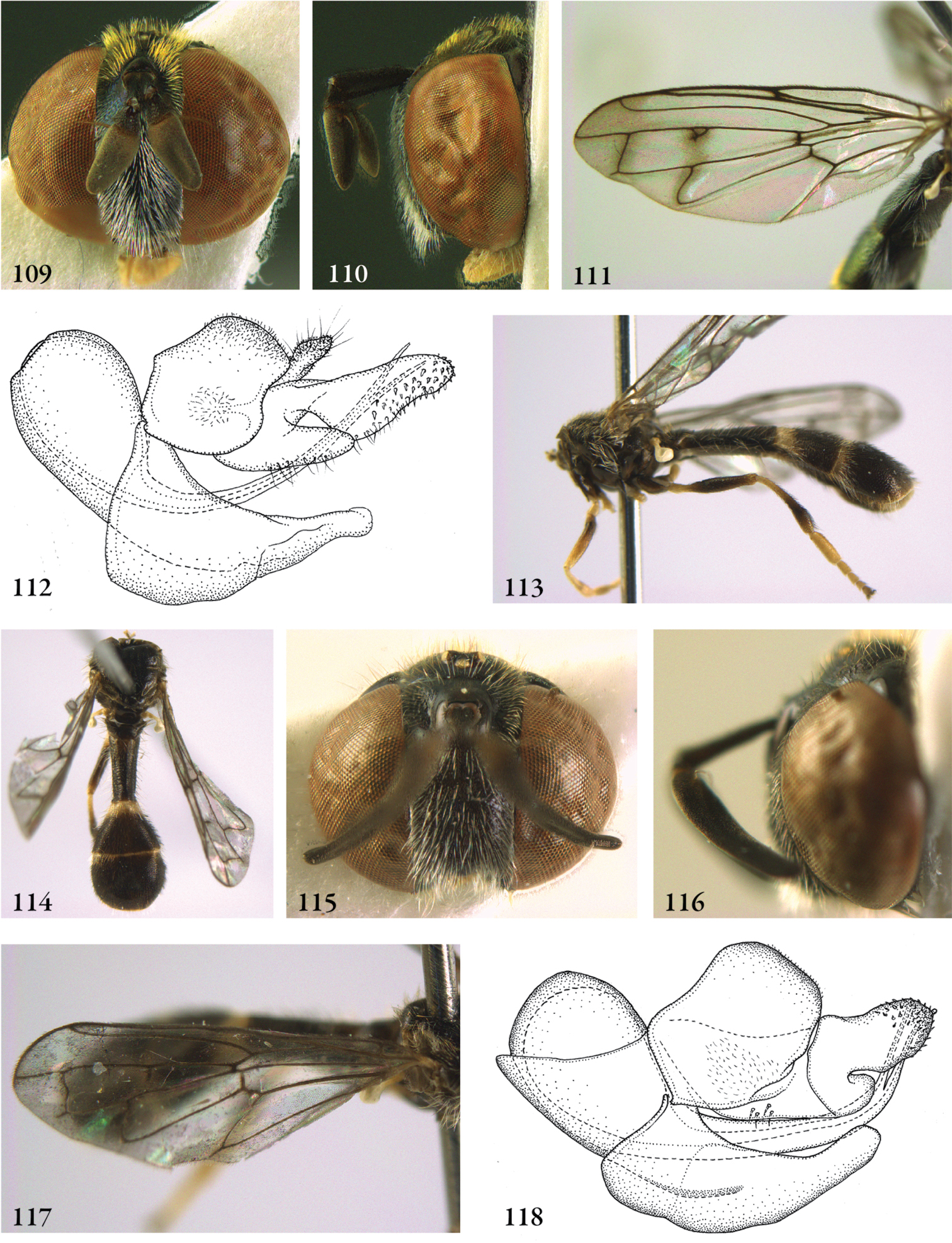
Description. Body length (intended only as an approximation, as not all specimens have been measured). A short characterization of the habitus is given, followed by a general description, which is intended to give characters considered (potentially) useful for identification, and to indicate the variability of characters. Unless stated otherwise, all listed characters apply to both sexes. Illustrations are given to illustrate habitus, important external characters and male genitalia. Additional morphological characters can be found in the character matrix of Reemer and Ståhls (in press).
Diagnosis. The shortest possible enumeration of external characters considered sufficient to distinguish the genus from all other Microdontinae. Characters of the male genitalia are only given in a few cases. The combination of the given characters is necessary for the diagnosis; all characters not given are considered unnecessary for this purpose. In some cases this diagnosis will not add much to the characters given in the key, but in other cases it will provide a ‘short-cut’ to the recognition of the genus.
Discussion. Arguments are given for the proposed classification. Other comments are given when necessary, e.g. on type specimens, history of classification, and morphological characters.
Diversity and distribution. The number of described species is given, sometimes with a speculation on the possible number of undescribed species. When available, a reference to species keys is given. The known geographic range is indicated.
Etymology. Only given for newly described genera.
http://species-id.net/wiki/Afromicrodon
Figs 3–6Body length: 6–9 mm. Relatively small flies with short antennae and oval abdomen. Head slightly wider than thorax. Face evenly convex; narrower than an eye. Lateral oral margins not produced. Vertex flat. Occiput narrow over entire length. Eye bare. Eyes in male strongly approaching each other at level of frons; with mutual distance about equal to width of antennal fossa. Antennal fossa about as high as wide. Antenna shorter than distance between antennal fossa and anterior oral margin. Basoflagellomere approximately as long as scape; oval, short; bare. Postpronotum pilose. Anepisternum without sulcus; pilose, except bare on ventral 1/4. Anepimeron pilose on dorsal half, bare on ventral half. Katepimeron convex; bare. Scutellum semicircular; without calcars. Wing: vein R4+5 without appendix; vein M1 anterior half directed somewhat outward, making acute angle with R4+5, posterior half perpendicular to vein M; crossvein r-m located around basal 1/4 of cell dm. Abdomen oval. Male genitalia: phallus straight, not furcate; hypandrium with bulb-like base and basolateral bulges; epandrium without ventrolateral ridge; surstylus large: about as long as hypandrium, somewhat sickle-shaped.
Vertex flat. Occiput narrow. Antenna shorter than distance between antennal fossa and anterior oral margin. Postpronotum pilose. Katepimeron bare. Vein R4+5 without posterior appendix. Abdomen oval.
Described species: 5. Restricted to Madagascar and the Comorean islands.
http://species-id.net/wiki/Archimicrodon
Figs 7–26Body length: 4–11 mm. Small to moderately sized flies with short antennae and oval abdomen. Head about as wide as thorax or slightly wider. Face convex; narrower than an eye. Lateral oral margins not produced. Vertex flat. Occiput ventrally narrow, dorsally widened. Eye bare. Eye margins in male strongly converging at level of frons, with mutual distance about as large as width of antennal fossa. Antennal fossa about as wide as high. Antenna shorter than distance between antennal fossa and anterior oral margin; basoflagellomere as long as or longer than scape, oval, sometimes with acute apex and concave dorsal margin; bare. Postpronotum pilose. Scutellum semicircular; with or without calcars, sometimes apicomedially sulcate; in subgenus Hovamicrodon calcars are spatulate (spoon-shaped). Anepisternum weakly sulcate; pilose anteriorly and posteriorly, widely bare in between. Anepimeron entirely pilose. Katepimeron convex; bare. Wing: vein R4+5 with or without posterior appendix (this appendix only lacks in certain undescribed species from New Guinea); vein M1 perpendicular to vein R4+5; postero-apical corner of cell r4+5 rectangular, with small appendix; crossvein r-m located around basal 1/5 to 1/4 of cell dm. Abdomen oval, about 1.5 to 2 times as long as wide. Tergites 3 and 4 fused. Sternite 1 pilose or bare. Male genitalia: phallus furcate, with furcation point near apex; hypandrium with basal part bulb-like; epandrium without ventrolateral ridge; surstylus unfurcate.
Abdomen oval. Antenna shorter than distance between antennal fossa and anterior oral margin. Postpronotum pilose. Postero-apical corner of cell r4+5 rectangular. Proepimeron bare. Anepisternum widely bare medially, also on dorsal half. Anepimeron entirely pilose. Vein R4+5 usually with posterior appendix; if not: thorax and abdomen entirely black.
Archimicrodon was described as a subgenus of Microdon by Hull (1945), and considered as such by subsequent authors, including Cheng and Thompson (2005), who stated that three species are included. Most of the species assigned to Archimicrodon in the present paper were previously placed in Microdon. However, they do not agree with the more strict definition of Microdon used here. Its independent position from Microdon and its monophyly are supported by the phylogenetic results of Reemer and Ståhls (in press). These are reasons to raise Archimicrodon to generic status.
Three groups are recognized within this genus: Archimicrodon s.s., the subgenus Hovamicrodon, and a ‘leftover’ group, here called Archmicrodon s.l. Archmicrodon s.s. is based on Archmicrodon simplicicornis (de Meijere, 1908), a subjective senior synonym of the type species of the genus, Microdon digitator Hull, 1937 syn. n. Archimicrodon s.s. is here defined by the shape of the surstylus: more or less oval, without a long posterior process (Fig 9, 15); scutellar calcars are either present or absent, but never spatulate. The subgenus Hovamicrodon is defined (following Keiser 1971) by the spatulate shape of the scutellar calcars (Fig. 18); the surstylus has a long posterior process (Fig. 19). Archimicrodon s.l. is here defined as containing all other species, in which the scutellar calcars are absent or - if present - not spatulate, and in which the surstylus has a long posterior process (Figs 22–26). As far as the African species are concerned, this group corresponds with the brevicornis-group of Bezzi (1915).
The three groups are very similar in their morphology, except for the small differences as noted above. It seems likely that the groups are closely related. The subgenus Hovamicrodon is probably monophyletic, considering the spatulate scutellar calcars in combination with its restricted distribution (Madagascar). However, as the phylogenetic analyses by Reemer and Ståhls (in press) indicate, it is so closely related to Archimicrodon s.l. (which is recovered as paraphyletic with respect to Hovamicrodon) that a separate generic status seems not warranted. Besides, a spatulate shape of the scutellar calcars can also be found in certain species of the New World genera Laetodon gen. n. and Serichlamys Curran, 1925. The latter genus is recovered as sister to Archimicrodon by Reemer and Ståhls (in press). As this character is not unique, it does not provide sufficient basis to base a genus on.
Sexual dimorphism can be pronounced, especially in the African species of this group (including Hovamicrodon). Females tend to be much larger than males, and are different in colouration (usually darker). As several species were described from one sex only (such as certain Madagascar species described by Keiser 1971), it is possible that some of these species are actually synonyms. However, as many taxa are represented by only one specimen, these matters cannot yet be resolved.
Hova is the name of one of the social castes of the Merina, an ethnic group indigenous to Madagascar. Keiser (1971) used this name for his genus Hovamicrodon. Surprisingly, he did not include the Madagascar species Microdon hova Hervé-Bazin, 1913 in this genus, although this species clearly belongs to this group (spatulate scutellar calcars). Keiser (1971) does mention a specimen which he believes to be Microdon hova, based on the description, but for some reason this species is not listed under Hovamicrodon. However, when Keiser died in 1969, his paper was not finished yet. It was published posthumously, after the manuscript was finished and submitted by E. Lindner. Therefore, it is seems possible that Keiser intended to include Microdon hova in Hovamicrodon.
In genitalia, Microdon browni Thompson, 1968 is similar to Archimicrodon s.l.: phallus short, apically furcate, with dorsobasal projection; hypandrium with bulb-like base; surstylus with two elongate lobes; epandrium without ventrolateral ridge. In external morphology, the only difference with Archimicrodon seems to be that the antennae are longer than the distance between the antennal fossa and the anterior oral margin. This character is considered not important enough for group definition, as antennal length is quite variable within many genera of Microdontinae. For these reasons, Microdon browni is here considered as a species of Archimicrodon s.l. The phylogenetic analysis of morphological characters by Reemer and Ståhls (in press) provides no further clue to the taxonomic affinities of this taxon.
Described species: 45. Widely distributed in the Afrotropical, Oriental and Australasian regions, with one species known from the Eastern Palaearctic (Archimicrodon simplex (Shiraki, 1930)). Archimicrodon s.s. is only known from the Oriental region. The number of species of Archimicrodon s.s. is not known, as the male genitalia of several species were not studied. The subgenus Hovamicrodon (six species) is restricted to Madagascar.
http://species-id.net/wiki/Aristosyrphus
Figs 27–34Aristosyrphus (Aristosyrphus). Body length: 6–18 mm. Slender flies, often with constricted abdomen. Head wider than thorax. Face convex or almost straight in profile; about as wide as an eye or narrower. Lateral oral margins not produced. Vertex flat. Occiput narrow over entire length. Eye bare. Eyes in male weakly converging at level of frons, with mutual distance 2 to 3 times the width of antennal fossa. Antennal fossa about as wide as high. Antenna longer or shorter than distance between antennal fossa and anterior oral margin; basoflagellomere longer than scape, oval; bare. Postpronotum pilose. Scutellum semicircular; without calcars. Anepisternum without or with weak sulcus; anteriorly pilose or bare, posteriorly pilose, with pile limited to dorsal half. Anepimeron entirely pilose. Katepimeron convex; bare. Wing: vein R4+5 without posterior appendix; vein M1 making acute angle with vein R4+5, anterior part or entire vein M1 parallel to wing margin; postero-apical corner of cell r4+5 angular, with small appendix; crossvein r-m located within basal 1/7 of cell dm, often very close to base. Abdomen elongate: slightly oval, parallel-sided or constricted at segment 2; more than twice as long as wide. Tergites 3 and 4 fused. Male genitalia: phallus unfurcate, straight or bent dorsad; ejaculatory hood apicodorsally separately developed from actual phallus into prong-like structure, which may be mistaken for dorsal aedeagal process, but does not contain a sperm-duct; apical part of hypandrium consists of two separate lobes (separated ventromedially); epandrium without ventrolateral ridge; surstylus furcate or unfurcate.
Aristosyrphus (Eurypterosyrphus) Body length: 8-14 mm. Slender flies with parallel-sided, constricted or kite-shaped abdomen. Head wider than thorax. Face more or less straight, with median tubercle on dorsal half; about as wide as an eye or narrower. Vertex flat. Occiput narrow over entire length. Eye bare. Eyes in male not or only slightly converging at level of frons, with mutual distance 4 to 5 times the width of antennal fossa. Antennal fossa about as wide as high. Antenna longer than distance between antennal fossa and anterior oral margin; basoflagellomere shorter or longer than scape, oval, sometimes appearing swollen: more than twice as wide as scape; bare. Postpronotum pilose. Scutellum semicircular; without calcars. Anepisternum without or with weak sulcus; pilose on dorsal half, bare ventrally. Anepimeron pilose on dorsal half, bare ventrally. Katepimeron convex; bare. Wing: vein R4+5 without posterior appendix; vein M1 making straight or acute angle with vein R4+5; postero-apical corner of cell r4+5 angular, with small appendix; crossvein r-m located around basal 1/3 of cell dm. Abdomen parallel-sided, constricted or kite-shaped; more than twice as long as wide. Tergites 3 and 4 fused. Male genitalia: phallus unfurcate, straight or bent dorsad; ejaculatory hood apicodorsally enveloping phallus; apical part of hypandrium consists of two separate lobes (separated ventromedially); hypandrium in some species with elongate ventromedian structure parallel to phallus Figs 32, 34), resembling the lingula of certain taxa of the subfamily Syrphinae; epandrium without ventrolateral ridge; surstylus furcate or unfurcate.
Vein R4+5 without posterior appendix. Abdomen elongate and parallel-sided or constricted. Postpronotum pilose. Mesoscutum with transverse suture incomplete. Antenna longer than distance between antennal fossa and anterior oral margin.
Aristosyrphus s.s. Vein M1 oblique, at least anterior half parallel to wing margin. Face evenly convex. Anepimeron entirely pilose. Crossvein r-m located around basal 1/3 of cell dm. Ejaculatory hood apicodorsally developed into prong-like structure, separate from actual phallus (phallus may seem furcate under casual observation, but ejaculatory hood does not contain sperm duct).
Eurypterosyrphus. Vein M1 oblique or straight. Face with median tubercle. Anepimeron bare on ventral half. Crossvein r-m located within basal 1/7 of cell dm. Ejaculatory hood apicodorsally enveloping phallus, not developed into separate, prong-like structure.
Morphological variation within this group is large, especially in the male genitalia (Figs 29, 32–34). In some specimens of Aristosyrphus primus Curran, 1941 an anterior stump is present at vein M (Fig. 28). This character has always been used as diagnostic for Mixogaster (Hull 1954, Cheng and Thompson 2008).
Described species: 7 (Aristosyrphus s.s.: 4; Eurypterosyrphus: 3). Several undescribed species are known to the first author. Central and South America.
http://species-id.net/wiki/Bardistopus
Figs 35–37Body length: 6–7 mm. Small, dark flies with very long antennae and oval abdomen, which in lateral view appears constricted. Head slightly wider than thorax. Face evenly convex. Lateral oral margins not produced. Vertex flat. Occiput ventrally narrow; dorsally slightly widened. Eye bare. Eyes in male not converging at level of frons; mutual distance much larger than width of antennal fossa. Antennal fossa about as high as wide. Antenna longer than distance between antennal fossa and anterior oral margin. Basoflagellomere about six times as long as scape. Postpronotum bare. Scutellum semicircular; without calcars. Anepisternum without sulcus; pilose anteriorly and posteriorly, widely bare in between. Anepimeron pilose on dorsal half, bare on ventral half. Katepimeron flat; pilose. Wing: vein R4+5 with posterior appendix; vein M1 perpendicular to vein R4+5 and vein M; crossvein r-m located around basal 1/7 of cell dm. Abdomen oval in dorsal view, but in lateral view appearing constricted due to flattened segment 2. Tergites 3 and 4 fused. Male genitalia: phallus furcate, with furcation point in apical half, strongly bent dorsad; epandrium without ventrolateral ridge; surstylus elongate, bent dorsad.
Vein R4+5 with posterior appendix. Postpronotum bare. Abdomen in dorsal view oval; in lateral view constricted at segment 2. Basoflagellomere about six times as long as scape.
No statements about taxonomic affinities of Bardistopus have been made previously, except Cheng and Thompson (2008) who wrote that the name was ‘established for a Microdon species, which has a greatly elongate basoflagellomere’. Clearly the taxon does not belong to Microdon, because of the long basoflagellomere and the structure of the male genitalia (apically furcate phallus). These characters, combined with the bare postpronotum, suggest it might be related to Paramixogaster. However, the phylogenetic analysis of morphological characters by Reemer and Ståhls (in press) place it as a sister group of a clade containing taxa of which the male has a furcate basoflagellomere: Schizoceratomyia, Furcantenna and Carreramyia. Future studies employing molecular data could help elucidate the phylogenetic affinities of Bardistopus.
According to Mann (1920) the type specimens of the type species are females, but actually both are males (coll. USNM).
Described species: 1. Solomon Islands: Ugi.
http://species-id.net/wiki/Carreramyia
Figs 38–41Body length: 5–8 mm. Yellowish brown or black flies, tergites sometimes yellow with dark vittae. Mimics of stingless, Trigona-like bees (Apidae: Meliponini), due to the brush-like pilosity of the hind tibiae and the more or less triangular abdomen. Head wider than thorax. Face more or less straight in profile; wider than eye. Lateral oral margins not produced. Vertex strongly produced. Occiput ventrally narrow, dorsally widened. Eye bare. Eyes in male not approaching each other; separated over distance much wider than antennal fossa. Antennal fossa about as high as wide. Antenna longer than distance between antennal fossa and anterior oral margin. Antenna inserted below dorsal eye margin; basoflagellomere at least four times as long as scape, bifurcate in male, unfurcate in female; bare. Postpronotum pilose. Anepisternum without sulcus; continually pilose on dorsal half, bare on ventral half. Anepimeron pilose on dorsal half, bare on ventral half. Katepimeron convex; bare. Wing: vein R4+5 without posterior appendix; vein M1 perpendicular to R4+5 and M; crossvein r-m located close to bm-cu. Abdomen more or less triangular, with tergites 3 and 4 narrower than tergite 2. Tergites 3 and 4 fused. Sternite 1 bare or pilose. Male genitalia: phallus straight, furcate near apex; hypandrium with bulb-like base and basolateral bulges; epandrium without ventrolateral ridge.
Hind tibia widened and with long, brush-like pilosity. Vein R4+5 without posterior appendix. Vertex strongly produced but not shining and convex. Basoflagellomere at least four times as long as scape, bifurcate in male.
Described species: 2. Only the type species, Carreramyia megacephalus (Shannon, 1925), is known from more than one specimen (Panama and Costa Rica). The other species was found in Peru. Descriptions of two additional species from Peru and Surinam are in preparation by the first author. Apparently the genus is widespread in the Neotropical region.
http://species-id.net/wiki/Ceratophya
Figs 42–45Body length: 7–9 mm. Relatively small, black and yellow flies with long antennae and oval abdomen. Face in profile straight, with anterior oral margin somewhat produced ventrad; laterally depressed, therefore slightly carinate medially; somewhat wider than an eye. Lateral oral margins not produced. Vertex flat. Occiput narrow ventrally, slightly widened dorsally. Eye bare. Eyes in male not approaching each other, eye margins parallel; mutual distance much larger than width of antennal fossa. Antennal fossa about as high as wide. Antenna longer than distance between antennal fossa and anterior oral margin; basoflagellomere longer than scape; elongate, oval. Postpronotum pilose. Anepisternum with shallow sulcus; entirely short pilose, except bare on ventral 1/4. Anepimeron entirely pilose. Katepimeron weakly convex; bare. Scutellum semicircular or apicomedially sulcate; without calcars. Wing: vein R4+5 with posterior appendix; vein M1 perpendicular to vein R4+5 and vein M. Legs: hind tibia somewhat swollen; hind metatarsus enlarged, quadrate, sometimes with strong basoventral tooth. Abdomen with tergite 4 in lateral view more or less perpendicular to tergite 2. Tergites 3 and 4 not fused, able to articulate independently; in female with posterior margin of tergite 3 strongly overlapping tergite 4. Male genitalia: phallus strongly bent dorsally, furcate basally, with ejaculatory hood dorsally strongly elongate and thus forming a third process about equally long as two aedeagal processes; epandrium with ventrolateral ridges.
Tergites 3 and 4 not fused, strongly overlapping. Tergite 4 in lateral view more or less perpendicular to tergite 2. Basoflagellomere bare; longer than scape.
Cheng and Thompson (2008) point out the confused taxonomic history of Ceratophya. Unlike these authors, who consider the group as a subgroup of Microdon, it here treated as a separate genus. This is done because of the phylogenetic results of Reemer and Ståhls (in press) and because it does not agree with the diagnosis of Microdon as defined in the present paper.
http://species-id.net/wiki/Ceratrichomyia
Figs 46–58Body length: 7–10 mm. Slender, black flies with yellow markings and a constricted abdomen. Head wider than thorax, face and vertex wider than an eye. Face ventrally produced in profile; wider than an eye. Lateral oral margins not produced. Vertex swollen. Occiput narrow ventrally, strongly widened dorsally. Eye bare. Eyes in male not approaching each other; smallest mutual distance much larger than width of antennal fossa. Antennal fossa about as high as wide. Antenna longer than height of head. Basoflagellomere at least three times as long as scape; with long pilosity. Postpronotum pilose or bare. Mesoscutum with transverse suture complete. Scutellum without calcars. Anepisternum with deep sulcus; entirely pilose. Anepimeron entirely pilose. Katepimeron convex; pilose or bare. Wing: vein R4+5 with posterior appendix; vein M1 straight, perpendicular to R4+5 and M; postero-apical corner of cell r4+5 rectangular, with small appendix; crossvein r-m located around basal 1/4 of cell dm. Abdomen constricted at segment 2. Tergites 3 and 4 not fused, able to articulate independently. Sternite 1 bare. Sternite 4 in male covered by genital capsule, therefore not visible without removing genitalia. Male genitalia: phallus straight or slightly bent dorsad, with spherical base very large, at least as long as remaining part of phallus; phallus furcate near apex; epandrium with or without ventrolateral ridge; surstylus deeply furcate.
Diagnosis. The combination of a complete transverse suture on the mesoscutum and a constricted abdomen is only found in Ceratrichomyia, Indascia Keiser, Indascia, 1958, Thompsodon gen. n. and certain species of Paramixogaster Brunetti, 1923. Males are easily distinguished from all these taxa by the long pilosity of the basoflagellomere, and also by sternite 4, which is covered by the genital capsule. From Paramixogaster this genus also differs by the unfused tergites 3 and 4. Females are unknown.
Discussion. Séguy (1951) attributed one species to this genus. He designated a male and a female as ‘types’, and another male as ‘cotype’. These are here all considered as syntypes. Examination of these three specimens made clear that they belong to three different species, which makes it necessary to designate a lectotype. The male with the following label data is here designated as lectotype. Label 1: “Madagascar, Behara”; label 2 (blue): “Museum Paris, III-38, A. Seyrig”; label 3 (red): “Type”; label 4: “Ceratrichomyia behara type du genre [male symbol] Séguy 50”; coll. MNHN. A redescription of the lecotype is given in the next section of the present paper. By this lectotype designation, the other two syntypes become paralectotypes. The male collected in Bekily (Madagascar) belongs to a new species of Ceratrichomyia, which is described in the present paper as Ceratrichomyia bullabucca spec. n. The female paralectotype, collected in Bekily, is here considered to belong to a previously undescribed species of Paramixogaster, because it possesses all characters described as diagnostic for that genus (see genus account). A description of that species is given under the name Paramixogaster piptotus sp. n. A third species attributed to this genus, Ceratrichomyia angolensis sp. n., is described from Angola.
The long pilosity of the male basoflagellomere was used by Séguy (1951) as a character to set his African genus Ceratrichomyia apart from other Microdontinae. This character is also present in Ptilobactrum Bezzi, 1915, another African taxon. Apparently Séguy was not aware of this, as he did not refer to Ptilobactrum. Cheng and Thompson (2008) did notice the similarity in antennal structure in both taxa and, based on the descriptions, proposed to regard Ceratrichomyia as a subjective junior synonym of Ptilobactrum.
Study of the type specimens of Ceratricomyia and Ptilobactrum revealed that these taxa are in fact very different. While Ceratrichomyia has, for instance, a constricted abdomen with unfused tergites 3 and 4, Ptilobactrum has a conical abdomen with fused tergites 3 and 4. The structures of the male genitalia are also very different (compare Figs 56–58 with 326), e.g. with a deeply furcate surstylus in Ceratrichomyia and an unfurcate one in Ptilobactrum. Considering these morphological differences, and supported by the phylogenetic results of Reemer and Ståhls (in press), Ceratrichomyia is here re-instated as a valid genus.
Diversity and distribution. Described species: 3. Two species are known from Madagascar, one from the African mainland (Angola).
http://species-id.net/wiki/Ceriomicrodon
Figs 59–60Body length: 11 mm. Very slender, wasp-like flies with long antennae and constricted abdomen. Face convex, somewhat produced on ventral half; narrower than an eye. Lateral oral margins clearly produced. Vertex flat. Occiput ventrally narrow, dorsally somewhat widened. Eye bare; frontally with narrow, horizontal area of enlarged ommatidia at level of antenna. Eyes in male strongly convergent at level of frons. Antennal fossa about 1.5 times as wide as high. Antenna longer than height of head; basoflagellomere more than twice as long as scape; bare. Postpronotum bare. Anepisternum with shallow sulcus; pilose along posterior margin and sparsely anterodorsally, widely bare in between. Anepimeron entirely pilose. Katepimeron flat; bare. Scutellum semicircular; without calcars. Wing: vein R4+5 with posterior appendix; vein M1 perpendicular to vein R4+5; postero-apical corner of cell r4+5 widely rounded; crossvein r-m located around basal 1/3 of cell dm. Abdomen very slender, constricted at tergite 2. Tergite 2 longer than thorax, about as long as tergites 3-5 together. Tergites 3 and 4 fused. Male genitalia: phallus furcate near apex, with dorsal process long and whip-like, ventral process very short; epandrium with ventrolateral ridge.
Postpronotum bare. Vein R4+5 with posterior appendix. Postero-apical corner of cell r4+5 widely rounded. Abdomen constricted. Tergite 2 longer than thorax.
Ceriomicrodon is treated as a subgenus of Microdon by Thompson et al. (1976) and Cheng and Thompson (2008). However, it does not agree with the diagnosis of Microdon as used in the present paper, because of several characters (e.g. postpronotum bare, abdomen petiolate, phallus with dorsal process long and whip-like). In addition, the phylogenetic results of Reemer and Ståhls (in press) indicate a relationship with e.g. Pseudomicrodon and Rhopalosyrphus.
Described species: 1. Known from Central (Mato Grosso) and Northern Brazil (Roraima).
http://species-id.net/wiki/Cervicorniphora
Figs 61–62Body length: 8 mm. Broadly built flies with oval abdomen. Head wider than thorax. Face convex in profile; wider than an eye. Lateral oral margins not produced. Antennal fossa about as wide as high. Vertex flat. Occiput rather wide, dorsally strongly widened. Eye bare. Eye margins in male not converging at level of frons; with mutual distance about five times the width of antennal fossa. Antenna longer than distance between antennal fossa and anterior oral margin; basoflagellomere longer than scape, bare, bifurcate, with dorsal branch narrower and shorter than ventral branch, ventral branch strongly curved; arista well-developed. Postpronotum pilose. Scutellum semicircular; without calcars. Anepisternum moderately sulcate; pilose anteriorly and posteriorly, bare medially. Anepimeron entirely pilose. Katepimeron convex; bare. Wing: vein R4+5 with posterior appendix; vein M1 perpendicular to vein R4+5; postero-apical corner of cell r4+5 widely rounded; crossvein r-m located around basal 1/4 of cell dm. Abdomen oval, about 1.5 times as long as wide. Tergites 3 and 4 fused. Male genitalia: phallus unfurcate; epandrium without ventrolateral ridge; surstylus with long posterior process and wide anterior lamella. Female unknown.
Basoflagellomere bifurcate. Vein R4+5 with posterior appendix.
Although Ferguson (1926a) argued that the furcate antenna provides insufficient basis for erecting a new genus for Microdon alcicornis, Hull (1945) decided to erect Cervicorniphora for this species, as a subgenus of Microdon. Cheng and Thompson (2008) also considered this genus-group as a subgenus of Microdon. The phylogenetic analysis of morphological characters by Reemer and Ståhls (in press) did not provide many clues as to the taxonomic affinities of this taxon, although it seems clear that it is not related to other taxa in which the male has a furcate basoflagellomere. As the characters of Cervicorniphora (e.g. phallus not furcate) do not fit in the concept of Microdon s.s. (phallus furcate near base) as defined in the current paper, Cervicorniphora is here raised to genus rank, to avoid disrupting the monophyly of Microdon.
The female is unknown. In most other microdontine taxa in which the male has a furcate basoflagellomere (e.g. Carreramyia, Schizoceratomyia), the female has an unfurcate basoflagellomere. So, the possibility that the female of Cervicorniphora has unfurcate antennae should be taken into account.
Described species: 1. Australia: New South Wales, Queensland and Tasmania (Ferguson 1926a).
http://species-id.net/wiki/Chrysidimyia
Figs 63–67Body length: 8–10 mm. Metallic green to bluish flies (legs may be yellowish), entire body densely and coarsely punctate, mimics of Chrysididae (Hymenoptera). Head about as wide as thorax. Face convexly produced in profile; about as wide as an eye. Lateral oral margins produced. Vertex flat. Occiput ventrally narrow, dorsally strongly widened. Eye densely pilose. Eyes in male with mutual distance smaller than width of antennal fossa. Antennal fossa twice as wide as high, dorsally covered by ‘shelf-like’ extension of frons. Antenna longer than distance between antennal fossa and anterior oral margin; basoflagellomere longer than scape, oval; bare. Postpronotum pilose. Notal wing lamina strongly developed; partly overlapping membranes around wing insertion. Scutellum semicircular; with calcars. Anepisternum moderately sulcate; with bare part limited to ventral half. Anepimeron entirely pilose. Katepimeron flat; bare. Katatergum carinate. Wing: vein R4+5 with posterior appendix; vein M1 perpendicular to vein R4+5; postero-apical corner of cell r4+5 widely rounded; crossvein r-m located around basal 1/4 of cell dm. Abdomen oval, about 1.5 times as long as wide. Posterior margin of tergite 1 angular. Tergites 3 and 4 fused. Male genitalia: phallus unfurcate; epandrium without ventrolateral ridge; surstylus furcate, with anterior part short and wide, posterior process long and narrow.
Head, thorax and abdomen metallic green or blue. Antennal fossa twice as wide as high, dorsally covered by ‘shelf-like’ extension of frons.
Chrysidimyia was treated as a synonym of Microdon by Thompson et al. (1976), but the unfurcate phallus and the phylogenetic results of Reemer and Ståhls (in press) indicate that this status cannot be maitained. Instead, the male genitalia of Chrysidimyia (Fig. 65) resemble those of Laetodon (Fig. 135); these taxa share an unfurcate phallus and a long posterior process on the surstylus. These taxa also have their metallic body colouration and pilose eyes in common. These characters may suggest a phylogenetic relationship, although this is not found by Reemer and Ståhls (in press), who recovered Chrysidimyia in a large polytomy. Besides, the ‘shelf-like’ extension of the frons and dense punctuation of the body are not found in Laetodon. For this reason, we prefer to treat the groups separately.
Described species: 1. One additional, undescribed species is known to the first author. All known records are from the Amazon region of South America, including the Guyana shield.
urn:lsid:zoobank.org:act:EB942C77-8F1C-4095-B98A-74BF58F6E810
http://species-id.net/wiki/Domodon
Figs 68–73Body length: 6–8 mm. Moderately small flies with short antennae and oval abdomen. Head a little wider than thorax. Face convex; about as wide as or narrower than an eye. Lateral oral margins weakly produced. Vertex convexly produced, more or less shining, sparsely pilose, almost bare on anterior half. Occiput ventrally narrow, dorsally widened. Eye bare. Eye margins in male weakly converging at level of frons, with mutual distance 3-5 times width of antennal fossa. Antennal fossa about as wide as high. Antenna longer than distance between antennal fossa and anterior oral margin; basoflagellomere as long as or longer than scape, bare. Postpronotum pilose. Scutellum semicircular; with calcars. Anepisternum sulcate; pilose anterodorsally and posteriorly, widely bare in between. Anepimeron entirely pilose. Katepimeron almost flat to convex; often with wrinkled texture; bare. Wing: vein R4+5 with posterior appendix; vein M1 perpendicular to vein R4+5; postero-apical corner of cell r4+5 rectangular, with small appendix; crossvein r-m located between basal 1/6 to 1/4 of cell dm. Abdomen oval, about 1.5 to 2 times as long as wide. Tergites 3 and 4 fused. Sternite 1 bare. Male genitalia: phallus furcate near apex, with dorsal process long and whip-like, ventral process very short; epandrium with ventrolateral ridge.
. Vertex convexly produced. Abdomen oval. Vein R4+5 with posterior appendix. Tergites 3 and 4 fused. Membrane between sternites 2 and 3 much less wide than sternite 2.
All species assigned to this genus were previously undescribed or are still undescribed.
The phylogenetic analysis based on morphology places the type species (Domodon zodiacus sp. n.) in the same clade as Omegasyrphus Giglio-Tos, 1891, Pseudomicrodon Hull, 1937 and Rhopalosyrphus Giglio-Tos, 1891 (Reemer and Ståhls in press). In addition to this phylogenetic evidence, the male genitalia of these taxa are all similar in the structure of the phallus and the shape of the surstylus. Because of the oval, non-constricted abdomen, Domodon species superficially may seem most similar to Omegasyrphus, but differ from that genus by the convex and sparsely pilose vertex, the long antenna, and the medially widely bare anepisternum. With Pseudomicrodon it shares the convex and sparsely pilose vertex, as well as the structure of the male genitalia, but Domodon differs from that genus by the oval (instead of constricted) abdomen.Instead of arbitrarily assigning the species in question to one of the mentioned genera, it is here considered preferable to erect a new genus, so as to emphasize the distinctive features of this group.
Described species: 1. Surinam. Four additional, undescribed species are known by the first author from French Guyana, Surinam and Costa Rica. Probably the group is widespread in Central and South America.
The generic name is a combination of domus and odon, with the latter used as a suffix derived from Microdon. The Latin word domus is here used in the meaning of ‘dome’ and refers to the convex (dome-shaped) vertex of the species in this genus. The name is to be treated as masculine.
http://species-id.net/wiki/Furcantenna
Figs 74–80Body length: 9–10 mm. Broadly built flies with very wide head, long antennae and widened hind tibiae, bee mimics. Head much wider than thorax. Face slightly convex in profile; wider than eye; laterally depressed; medially weakly carinate. Lateral oral margins not produced. Vertex produced. Occiput ventrally narrow, dorsally widened. Eye bare. Eyes in male not convergent at level of frons; separated over distance much larger than width of antennal fossa. Antennal fossa about as high as wide. Antenna much longer than height of head; basoflagellomere bifurcate at base, with ventral branch a little longer than dorsal branch, both branches entirely long pilose; arista absent. Postpronotum pilose. Anepisternum sulcate. Scutellum apicomedially sulcate. Katepisternum dorsally pilose. Metasternum developed and pilose. Wing: vein R4+5 without posterior appendix; vein M1 perpendicular to R4+5 and M; crossvein r-m located around basal 1/5 of cell dm. Hind tibia and tarsus widened. Abdomen oval. Male genitalia: phallus slightly bent dorsad, with large spherical base; phallus furcate near apex; epandrium without ventrolateral ridge; surstylus approximately oval. Females unknown.
Male with bifurcate basoflagellomere. Katepisternum pilose. Metasternum pilose.
Described species: 2. The type species was found in a mountainous area in southeastern China. The second known species, Furcantenna nepalensis sp. n., was collected in the Nepalese Himalaya at an altitude of approximately 1800 meters. The discovery of these species in these areas sheds an interesting light on the biogeography of the taxa with a furcate basoflagellomere in the male. Prior to the description of Furcantenna, such taxa were almost exclusively known from South America (except for the the apparently unrelated Australian Cervicorniphora). The occurrence of the obviously related (Reemer and Ståhls in press) Furcantenna in Oriental mountains on the Asian mainland could possibly be explained as a relict of a wider distribution in early eras.
urn:lsid:zoobank.org:act:6981D4FC-AE41-45D7-942D-10707E8045CE
http://species-id.net/wiki/Heliodon
Figs 81–96Microdon tricinctus de Meijere, 1908: 208. Type locality: Java.
Body length: 8–12 mm. Moderately slender to broadly built flies with long antennae; abdomen oval, slightly tapering or basally slightly constricted; often with fasciate patterns of golden pile on thorax and abdomen, sometimes with yellow abdominal markings. Head slightly wider or slightly narrower than thorax. Face convex; narrower than to as wide as an eye. Lateral oral margins produced. Vertex flat. Occiput ventrally narrow, dorsally widened. Eye short pilose or bare. Eye margins in male converging at level of frons, with mutual distance 1.5–2 times as large as width of antennal fossa. Antennal fossa about as wide as high. Antenna about as long as distance between antennal fossa and anterior oral margin; basoflagellomere shorter than scape; bare. Postpronotum pilose. Scutellum semicircular; with calcars. Anepisternum sulcate; entirely pilose, except for small bare part ventrally. Anepimeron entirely pilose. Katepimeron convex or nearly flat; with or without wrinkled texture; bare or pilose. Wing: vein R4+5 with posterior appendix; vein M1 more or less straight, perpendicular to vein R4+5; postero-apical corner of cell r4+5 rounded or rectangular, with or without small appendix; crossvein r-m located between basal 1/6 and 1/5 of cell dm. Abdomen oval or basally constricted, 1.5-3 times as long as wide. Tergites 3 and 4 fused. Sternite 1 pilose. Male genitalia: phallus projecting little beyond apex of hypandrium, bent dorsad, furcate with furcation point from halfway to near apex, with both processes about equally long; epandrium without ventrolateral ridge; surstylus with subbasal excavation, dividing surstylus into a basal lamella and a long posterior process.
Vein R4+5 with posterior appendix. Postpronotum pilose. Propleuron bare. Anepisternum almost entirely pilose, at most ventrally with small bare part. Mesonotum with transverse suture incomplete. Basoflagellomere shorter than scape. Tergite 1 long: length/width ratio 1:1.4 to 1:2. Tergite 2: anterior margin less than 1.5 times as wide as posterior margin. Body not entirely metallic green or blue.
All previously described species included in this genus were originally described in the genus Microdon. In the most recent catalogue of Oriental Microdontinae these species were listed under that genus (Knutson et al. 1975). As Microdon is defined more strictly in the present paper, the species can no longer be placed in that genus, hence a new genus is erected. Three new species are described in the present paper.
Described species: 8. Oriental, ranging from Sri Lanka to Thailand, Vietnam, Java and Borneo.
The generic name is composed of the Greek words helios (sun) and odon, with the latter part used as a suffix derived from Microdon. The first part was chosen to emphasize the Oriental (‘where the sun rises’) distribution of the genus.
http://species-id.net/wiki/Hypselosyrphus
Figs 97–102Body length: 7–10 mm. Stingless bee mimicking flies with short to moderately long antennae and oval to triangular abdomen. Head slightly wider than thorax. Face convex; narrower than an eye. Lateral oral margins not produced. Vertex narrow, in most species convexly produced and shining, flat in some species. Occiput narrow over entire length, except ventrally strongly widened in Hypselosyrphus ulopodus. Eye with short, sparse pile. Eye margins in male strongly converging at level of frons, with mutual distance smaller than width of antennal fossa, except 3 times as wide in Hypselosyrphus ulopodus. Antennal fossa about as wide as high. Antenna as long as or shorter than distance between antennal fossa and anterior oral margin; basoflagellomere shorter to longer than scape, oval; bare. Postpronotum pilose. Scutellum semicircular, triangular or apicomedially sulcate; without calcars. Anepisternum without or with weak sulcus; pilose anterodorsally and posteriorly, widely bare in between. Anepimeron entirely pilose. Katepimeron convex; bare. Wing: vein R4+5 without posterior appendix; vein M1 perpendicular to vein R4+5; postero-apical corner of cell r4+5 rectangular, with small appendix; crossvein r-m located between basal 1/8 to 1/4 of cell dm. Abdomen oval or kite-shaped, 1.2 to 2 times as long as wide. Tergites 3 and 4 fused. Sternite 1 pilose. Male genitalia: phallus furcate near apex, with dorsal process in some species a little longer than ventral process; epandrium with or without ventrolateral ridge.
Vein R4+5 without posterior appendix. Crossvein r-m located between basal 1/8 and 1/4 of cell dm. Subcostal vein joins costal vein after level of crossvein r-m. Postpronotum pilose. Abdomen oval or kite-shaped (tergite 2 wide, subsequent tergites triangularly narrowing). Antenna as long as or shorter than distance between antennal fossa and anterior oral margin. Basoflagellomere not furcate. Occiput narrow in dorsal half (usually also in ventral half, except in Hypselosyrphus ulopodus Hull, 1944).
When Hull (1937a) erected the genus Hypselosyrphus for his species trigonus, he mentioned its similarity to Ubristes Walker, 1852 species, without clearly stating the differences. In his key to the groups of Microdontinae, Hull (1949) separated these taxa by the absence (Hypselosyrphus) or presence (Ubristes) of an appendix on vein R4+5. This character serves well to separate Hypselosyrphus from Ubristes s.s. as defined in the present paper, and almost always for Stipomorpha (appendix on vein R4+5 seldomly missing), which was included in Ubristes until now. In later keys and catalogues, Hypselosyrphus was treated as a junior synonym of Ubristes (Thompson 1969, Thompson et al. 1976). Cheng and Thompson (2008) also considered Hypselosyrphus (and Stipomorpha Hull, 1945) synonymous with Ubristes, but nevertheless differentiated the groups in their key. They consider abdominal shape to be diagnostic: oval or rectangular in Ubristes, short, almost equilaterally triangular in Hypselosyrphus, much longer, isosceles triangular in Stipomorpha. As there are many varieties in abdominal shape among the the taxa involved, it is hard to decide where to draw the line. Other characters are necessary to distinguish these taxa satisfyingly (see key and diagnoses).
Described species: 11. Descriptions of five additional species are in preparation by the first author. Hypselosyrphus is known from Panama, the Amazon region and southern Brazil. Considering the small number of specimens known, it seems likely that the genus is widespread in tropical South America.
http://species-id.net/wiki/Indascia
Figs 103–118Body length: 4–10 mm. Small, slender flies with more or less constricted abdomen. Head wider than thorax. Face convex in profile; narrower than to wider than an eye. Lateral oral margins not produced. Vertex flat. Occiput ventrally narrow, dorsally strongly widened. Antennal fossa about as wide as high. Eye bare. Eye margins in male parallel, not converging at level of frons. Antenna shorter to longer than distance between antennal fossa and anterior oral margin. Basoflagellomere as long as to longer than scape, 1.5 to 5 times as long as wide; parallel-sided or with dorsal margin somewhat concave; bare. Postpronotum pilose. Mesoscutum with transverse suture complete. Scutellum semicircular, apex may be slightly acute; without or with very small calcars. Anepisternum convex or sulcate; entirely pilose or with bare part limited to ventral half. Anepimeron entirely pilose. Katepimeron (moderately) convex; bare. Wing: vein R4+5 with or without posterior appendix; vein M1 perpendicular to vein R4+5 and vein M; postero-apical corner of cell r4+5 rectangular, with small appendix; crossvein r-m located within basal 1/4 of cell dm, sometimes very close to base. Abdomen elongate, at least 3 times as long as wide; constricted, with narrowest point at posterior margin of tergite 2 and widest point at tergite 4. Tergites 3 and 4 not fused. Male genitalia: phallus furcate, with furcation point in distal half; epandrium without ventrolateral ridge; surstylus furcate, with anterior part short, posterior part about twice as long.
Abdomen constricted. Postpronotum pilose. Mesoscutum with transverse suture complete. Katepimeron bare. Frons laterally without concave area.
Originally this genus was included in the tribe Sphegini, as part of a subfamily Cheilosiinae (Keiser 1958). Thompson (1969) correctly recognized that it belongs to the Microdontinae, where it has remained since.
Originally, Indascia was based on two species with short antennae and without a posterior appendix on vein R4+5 (Keiser 1958). In two of the species included in the phylogenetic analyses of Reemer and Ståhls (in press) the antennae are long and the appendix on vein R4+5 is present (Indascia gigantica sp. n. and Indascia spathulata sp. n.). Both characters are also found in additional undescribed species known to the first author. Therefore, these characters are considered not to be of diagnostic value for this genus.
Superficially, species of Indascia look similar to those of Paramicrodon de Meijere, 1913 (as noticed by Cheng and Thompson 2008). For discussion on similarities with Paramixogaster Brunetti, 1923 see there.
Described species: 4. At least four undescribed species are known to the first author. The genus appears to be strictly Oriental, with species known from India, Sri Lanka, Pakistan, Thailand and Vietnam. The origin of the type specimens of the type species (‘India orientalis’) is not exactly known.
http://species-id.net/wiki/Kryptopyga
Figs 119–131Body length: 12–14 mm. Large flies with long antennae (pilose in male) and oval abdomen, which may be constricted basally. Head wider than thorax. Face in profile more or less straight, ventrally produced below eye margin; wider than eye. Lateral oral margins weakly produced. Vertex strongly swollen. Occiput narrow ventrally, strongly widened dorsally. Eye bare. Eyes in male not converging at level of frons; mutual distance about 5 times width of antennal fossa. Antennal fossa about as high as wide. Antenna longer than height of head. Basoflagellomere 3.5-4 (male) or 2.5 (female) times as long as scape; with long pilosity in male, bare in female. Postpronotum pilose. Mesoscutum with transverse suture incomplete. Scutellum semicircular, without calcars. Anepisternum with deep sulcus; pilose anterodorsally and posteriorly, widely bare in between. Anepimeron entirely pilose. Katepimeron convex; with or without wrinkled texture; with rows of microtrichia. Wing: vein R4+5 with posterior appendix; vein M1 in anterior half with outward angle; postero-apical corner of cell r4+5 rectangular, with small appendix; crossvein r-m located between basal 1/6 and 1/5 of cell dm. Abdomen either oval or somewhat constricted at base, in the latter case with tergite 4 curved downward and more or less perpendicular to tergite 2. Tergites 3 and 4 not fused, able to articulate independently. In male Kryptopyga pendulosa, sternite 4 is covered by the genital capsule and therefore not visible without removing genitalia, while the lateral margins of tergite 3 are strongly curved and ‘tucked away’ under sternite 3 (Fig. 123). Male genitalia: phallus slender, furcate near apex, basally complexly bent into curves, interconnected by a membrane; epandrium without ventrolateral ridge; surstylus approximately oval.
Vein R4+5 with posterior appendix. Postpronotum pilose. Propleuron bare. Mesonotal transverse suture incomplete. Tergites 3 and 4 not fused, able to articulate independently. Anepisternum widely bare of pile (but with microtrichia) medially, also on dorsal half. Male basoflagellomere with long pile.
Hull (1944a) erected the genus and assigned one species to it: Kryptopyga pendulosa Hull, 1944. He considered it close to the African genus Ptilobactrum Bezzi, 1915 because of the long pile on the basoflagellomere, but considered it distinct because of the subpetiolate abdomen and the remarkable structure of the 3rd and 4th abdominal segments. The pilose basoflagellomere in the male is also found in Ceratrichomyia, with which Kryptopyga also shares the swollen vertex and dorsal occiput, and the unfused tergites 3 and 4. The male genitalia, however, are quite different, and in Kryptopyga the mesonotal transverse suture is incomplete.
Together with the Nearctic Microdon craigheidi Walton, 1912, Kryptopyga is the only known taxon of Microdontinae in which the phallus is not simply curved between base and apex, but complexly bent into a couple of curves basally, interconnected by a membrane (compare Fig. 131 with Fig. 232). Despite this common character, there is no reason to suspect a closer relationship between these taxa.
The abdomen in Kryptopyga pendulosa is much more modified than in Kryptopyga sulawesiana sp. n., but the latter species is nevertheless regarded as belonging to the genus based on the pilose basoflagellomere, the shape of the head, the wing venation and the structure of the male genitalia, in which it is all very similar to Kryptopyga pendulosa.
Microdon tuberculatus Shiraki, 1968 might also belong in Kryptopyga, because of its unfused tergites 3 and 4 and similarity in head shape (strongly swollen vertex and dorsal occiput, face ventrally produced below eye margin). However, only the female of this species is known, so it is unknown whether the male has long pile on the basoflagellomere and the characteristic genitalia of Kryptopyga. Therefore, this species is presently left unclassified. As de Meijere (1913) had already used the same species name, the replacement name shirakii is here proposed.
Described species: 2. Indonesia: Bangka, Java and Sulawesi.
urn:lsid:zoobank.org:act:98DA55E3-2041-4F23-B5B8-141BDF62DEC5
http://species-id.net/wiki/Laetodon
Figs 132–135Body length: 6–9 mm. Small, metallic green to blue flies, with long antennae and oval abdomen. Head about as wide as thorax or slightly wider. Face convex; narrower than an eye. Lateral oral margins weakly produced. Vertex flat. Occiput ventrally narrow, dorsally widened. Eye pilose. Eye margins in male converging at level of frons, with mutual distance 2 to 4 times as large as width of antennal fossa. Antennal fossa about as wide as high. Antenna about as long as to longer than distance between antennal fossa and anterior oral margin; basoflagellomere longer than scape, oval; bare. Postpronotum pilose. Scutellum semicircular; with calcars, which may be spatulate (widened and dorsoventrally flattened). Anepisternum weakly sulcate; pilose anteriorly and posteriorly, widely bare in between. Anepimeron entirely pilose. Katepimeron convex; smooth; bare. Wing: vein R4+5 with posterior appendix; vein M1 perpendicular to vein R4+5; postero-apical corner of cell r4+5 rectangular, with small appendix; crossvein r-m located between basal 1/6 to 1/5 of cell dm. Abdomen oval, about 1.5 to 2 times as long as wide. Tergites 3 and 4 fused. Sternite 1 pilose or bare. Male genitalia: phallus unfurcate, projecting slightly beyond apex of hypandrium; hypandrium with basal part bulb-like; epandrium without ventrolateral ridge; surstylus shallowly furcate, with long posterior process.
Vein R4+5 with posterior appendix. Postpronotum pilose. Abdomen oval. Anepisternum widely bare medially. Propleuron pilose. Postero-apical corner of cell r4+5 rectangular. Eye pilose.
The species included in this genus used to be placed in Microdon (Thompson 1981b). Morphology of the male genitalia, however, is quite distinct from that of Microdon as defined in the present paper: the phallus is short and unfurcate, the epandrium lacks the ventrolateral ridge. Based on these morphological differences and the phylogenetic results of Reemer and Ståhls (in press), Laetodon is here erected as a new genus. See Chrysidimyia for discussion on possible relationships with that genus.
Described species: 5. Nearctic (4 species) and Neotropical (1 species).
http://species-id.net/wiki/Masarygus
Figs 136–150Body length: 4–7 mm. Small, delicate flies with long antennae and flat abdomen. Head slightly to much wider than thorax. Face concave, either entirely or only laterally; wider than an eye. Mouth parts undeveloped: oral opening absent or hardly visible. Vertex more or less flat, not strongly produced or convex. Occiput ventrally narrow or widened, dorsally widened. Eye bare. Eyes in male not converging at level of frons, with mutual distance about 4 times the width of antennal fossa. Antennal fossa about as wide as high or about 1.5 times as wide as high. Antenna as long as or longer than distance between antennal fossa and anterior oral margin; basoflagellomere longer than scape, multifurcate in male (3 to 14 branches), unfurcate in female; bare; arista absent in male, present in female. Postpronotum bare. Scutellum semicircular; without calcars. Anepisternum convex; entirely with sparse, bristle-like pile. Anepimeron bare or pilose. Katepimeron convex; bare; with or without wrinkled texture. Wing: vein R4+5 without posterior appendix; vein M1 perpendicular to vein R4+5; postero-apical corner of cell r4+5 widely rounded or rectangular, with or without small appendix; crossvein r-m located very close to base of cell dm (within basal 1/10). Abdomen dorsoventrally flattened; more or less trapezoid, with lateral margins gradually widening posteriad, with largest width at tergite 4; 1.5-2.5 times as long as wide. Tergites 3 and 4 fused. Male genitalia: phallus furcate near apex, straight, projecting not or hardly beyond apex of hypandrium; epandrium without ventrolateral ridge; surstylus unfurcate, more or less oval.
Vein R4+5 without posterior appendix. Postpronotum bare. Antenna at least as long as distance between antennal fossa and anterior oral margin. Antenna inserted on head above dorsal eye margin.
Originally, this genus was erected as the first known member of a new family, the Masarygidae (Brèthes 1908; but journal publication was 1909, see Sabrosky 1999). Brèthes associated it with Conopidae and Scenopinidae because of the wing venation, and with Oestridae because of the reduced mouthparts. He also noted a superficial resemblance to certain Stratiomyidae. Bezzi (1910) was the first to recognize Masarygus as belonging to the Syrphidae and related to Microdon, by pointing out its resemblance to Ceratophya and the apparent relationship with ants (as noted by Brèthes 1908). Shannon (1925) considered Masarygus as a synonym of Microdon. Brèthes (1928) objected by pointing out that Masarygus differs from Microdon in the distinct sexual dimorphism and also in wing venation. All subsequent authors have included Masarygus in the Microdontinae.
Masarygus was the first described syrphid taxon with a furcate basoflagellomere (in the male sex only). A few other taxa with this character were described during the 20th century: Schizoceratomyia Carrera, Lopes & Lane, 1947, Johnsoniodon Curran, 1947 and Carreramyia Doesburg, 1966. Masarygus, Schizoceratromyia and Johnsoniodon were considered synonymous by Hull (1949), who also regarded “Masarygus as a Rhoga with fissicorn antennae”, without explicitly including all of these taxa in Rhoga (the oldest name). Papavero (1962) also considered Masarygus, Schizoceratomyia and Johnsoniodon synonymous, because he found that the number of branches on the basoflagellomere (four in Masarygus planifrons, two in the other taxa) was a species-level character rather than a generic character. Van Doesburg (1966) did not agree and considered Masarygus and Schizoceratomyia (including Johnsoniodon) as distinct genera, because of distinct differences in shape of head, antenna and abdomen. Thompson et al. (1976) followed the opinion of Papavero (1962). Cheng and Thompson (2008) considered Masarygus and Schizoceratomyia as distinct groups.
Masarygus palmipalpus sp. n. is considered related to Masarygus planifrons because of the following shared characters: male basoflagellomere multifurcate; base of antenna in lateral view placed above dorsal eye margin; head strongly flattened; face concave; oral opening absent; abdomen dorsoventrally flattened; gradually widening hindward, with widest point at tergite 4; phallus furcate near apex, with both processes equally long.
In addition to Masarygus planifrons and Masarygus palmipalpus, two undescribed species are considered to belong to this genus. These species are included in the phylogenetic analyses of Reemer and Ståhls (in press) under the names Masarygus sp. 1 and sp. 2. The latter has three branches on the basoflagellomere, the first approximately 14. Whereas sp. 1 is placed in the same clade as Masarygus planifrons and Masarygus palmipalpus by Reemer and Ståhls (in press) (based on adult morphology), sp. 2 is placed in the clade containing Schizoceratomyia and Carreramyia. Species 2 is nevertheless included in Masarygus, because of the following characters: basoflagellomere multifurcate and bare (instead of bifurcate and pilose as in Schizoceratomyia); arista absent (present in Schizoceratomyia); base of antenna inserted on head above dorsal eye margin (not below as in Schizoceratomyia); vertex not strongly produced (in contrast with Carreramyia); crossvein r-m located within basal 1/10 of cell dm (between basal 1/4 and 1/8 in Schizoceratomyia); hind tibia not swollen and without long, brush-like pile (in contrast with Carreramyia). Unfortunately, the genitalia of the only known specimen of Masarygus species 2 are lost: there is a microvial containing postabdominal segments attached to the pin, but there are no genitalia in it.
Described species: 2. Neotropical. At least two undescribed species are known to occur (see Discussion). All species known so far, including the undescribed ones, have only been collected on one occasion.
urn:lsid:zoobank.org:act:56864290-24D2-4BEB-9FB8-5B96BCABAAFD
http://species-id.net/wiki/Menidon
Figs 151–156Body length: 5–10 mm. Small, broadly built flies with long antennae and short, almost round abdomen. Head about as wide as thorax. Face convex; slighly narrower to slightly wider than an eye. Lateral oral margins not produced. Vertex flat. Occiput ventrally narrow, dorsally widened. Eye bare. Eye margins in male parallel, not converging at level of frons, with mutual distance 4 times as large as width of antennal fossa. Antennal fossa about as wide as high. Antenna longer than distance between antennal fossa and anterior oral margin; basoflagellomere longer than scape, sickle-shaped; bare. Postpronotum pilose. Scutellum semicircular; with small calcars or only with pair of small tufts of black microtrichiae posteriorly. Anepisternum without sulcus; pilose on slightly less than dorsal half, bare on slightly more than ventral half. Anepimeron entirely pilose. Katepimeron convex; bare. Wing: vein R4+5 with posterior appendix; vein M1 perpendicular to vein R4+5; postero-apical corner of cell r4+5 rectangular, with small appendix; crossvein r-m located between basal 1/8 and 1/10 of cell dm. Abdomen approximately round, 1 to 1.2 times as long as wide. Tergites 3 and 4 fused. Sternite 1 bare. Male genitalia: phallus straight, furcate near apex, with both processes about equally long; hypandrium without apical part; epandrium without ventrolateral ridge; surstylus furcate, with anterior lobe small and narrow, posterior lobe larger and wider.
Basoflagellomere sickle-shaped: curved upward. Anepisternum bare on ventral half. Cell r4+5 with postero-apical corner rectangular. Sternite 1 bare.
This is the only one known taxon among the Microdontinae in which the apical part of the hypandrium is entirely lacking (Fig. 156). Among the Neotropical taxa, this taxon is unique in the sickle-shaped basoflagellomere. The latter character also occurs to some extent in some Nearctic (Microdon adventitus, Microdon globosus)and Old World taxa (some Archimicrodon, Myiacerapis, Oligeriops Hul, 1937), but these differ from Menidon in several other important characters, such as a furcate phallus (unfurcate in Oligeriops) and absence of apical part of hypandrium (present in all other Microdontinae). These morphological singularities, combined with the phylogenetic results of Reemer and Ståhls (in press) (sister of (Piruwa + Paramicrodon)), are reasons to place Microdon falcatus in its own genus. Thompson (2007) clarified the taxonomy of the type species, which has several synonyms.
Described species: 1. Central and South America. Unpublished molecular evidence suggests that more than one species is involved, but this needs further study.
The generic name is a combination of the Greek words mene (moon) and odon, with the latter used as a suffix derived from Microdon. The prefix meni- was chosen because of the crescent-shaped basoflagellomere in the type species.
urn:lsid:zoobank.org:act:D8DDC5FF-1258-4C11-895C-50208995F444
http://species-id.net/wiki/Mermerizon
Figs 157–162Mermerizon inbio spec. n. Type locality: Costa Rica.
Stingless bee mimicking flies with moderately long antennae and elongate oval abdomen. Head slightly wider than thorax. Face convex; narrower than an eye. Lateral oral margins not produced. Vertex flat. Occiput ventrally narrow, dorsally widened. Eye bare. Eye margins in male parallel, not converging at level of frons, with mutual distance 3–4 times as large as width of antennal fossa. Antennal fossa about as wide as high. Antenna shorter than (may be almost as long as) distance between antennal fossa and anterior oral margin; basoflagellomere slightly shorter to longer than scape, oval; bare. Postpronotum pilose. Scutellum semicircular; without calcars. Anepisternum without sulcus; pilose on dorsal half, bare on ventral half. Anepimeron pilose on dorsal half, bare on ventral half. Katepimeron convex; bare. Wing: vein R4+5 with posterior appendix; vein M1 perpendicular to vein R4+5; postero-apical corner of cell r4+5 rectangular, with small appendix; crossvein r-m located around basal 1/10 of cell dm. Abdomen oval, 2 to 3 times as long as wide. Tergites 3 and 4 fused. Sternite 1 pilose. Male genitalia: phallus slightly bent dorsad, furcate near apex, with dorsal process at least twice as long as ventral process; hypandrium with bulb-like base; epandrium without ventrolateral ridge.
Vein R4+5 with posterior appendix. Postero-apical corner of cell r4+5 rectangular, with small appendix. Postpronotum pilose. Propleuron bare. Membrane between sternites 2 and 3 less wide than sternite 2. Abdomen oval. Anepisternum bare on ventral half, pilose on dorsal half, except for small median bare part on dorsal half. Anepimeron bare on ventral half. (Male: eye margins parallel at level of frons, not converging).
The species of this genus are obvious mimics of stingless, Trigona-like bees in their tawny colouration and long pilose hind tibiae. At first sight they may be confused with Hypselosyrphus, Rhoga, or Stipomorhpa. From the first two genera, Mermerizon can be distinguished by the presence of a posterior appendix on vein R4+5, from Stipomorpha by the absence of a wide membrane between sternites 2 and 3. A presently undescribed Argentinian species lacks the long pilosity of the hind tibiae and does not seem to mimic these bees. Instead, it resembles Paragodon Thompson, 1969 and Surimyia Reemer, 2008 in general habitus, but is easily told apart by the presence of a posterior appendix on vein R4+5 and the male genitalia, which are very similar to those of the other two Mermerizon species.
Described species: 1. Descriptions of two additional species are in preparation by the first author. Neotropical (presently known from Costa Rica and Argentina).
The generic name is derived from the ancient Greek verb mermerizo, meaning ‘to deliberate’ or ‘to ponder’. This name was chosen because it took some deliberation before making the decision that a new genus was to be erected for the involved species. The name is to be treated as masculine.
urn:lsid:zoobank.org:act:A00DBDC3-F8BE-4A89-A421-04903BC81B1D
http://species-id.net/wiki/Metadon
Figs 163–175Microdon wulpii Mik, 1899: 143. Type locality: Indonesia, Sumatra. Replacement name for Microdon apicalis Wulp, 1892: 29 (preoccupied by Walker, 1858).
Body length: 7–21 mm. Slender to moderately broadly built flies with oval abdomen and long antennae. Head slightly wider than thorax. Face almost straight to convex in profile; narrower to wider than an eye. Lateral oral margins produced or not produced. Vertex flat. Occiput ventrally narrow, dorsally widened. Eye bare. Eye margins in male converging at level of frons, with mutual distance 2-3 times as large as width of antennal fossa. Antennal fossa about as wide as high. Antenna longer than distance between antennal fossa and anterior oral margin; basoflagellomere shorter than scape; bare. Postpronotum pilose. Scutellum semicircular; with or without calcars. Anepisternum sulcate; entirely pilose, except sometimes with small bare part ventrally (only known exception: Metadon bifasciatus, in which anepisternum is bare on entire ventral half). Anepimeron entirely pilose. Katepimeron flat to somewhat convex; smooth or with wrinkled texture; not pilose, but often with rows of microtrichia. Katatergum with oblique rows of microtrichia. Wing: vein R4+5 with posterior appendix; vein M1 more or less straight, perpendicular to vein R4+5; postero-apical corner of cell r4+5 angular to widely rounded, with or without appendix; crossvein r-m located between basal 1/7 and 1/4 of cell dm. Abdomen oval, 1.5-2.5 times as long as wide. Tergites 3 and 4 fused. Sternite 1 pilose. Male genitalia: phallus projecting not or little beyond apex of hypandrium (except projecting well beyond apex of hypandrium in Metadon bifasciatus), bent dorsad, furcate in apical half, with both processes about equally long (except ventral process much longer in Metadon bifasciatus); epandrium with or without ventrolateral ridge; surstylus unfurcate, sometimes with long posterior process.
Body never metallic green or blue. Vein R4+5 with posterior appendix. Abdomen oval, longer than wide but less than 2.5 times as long as wide. Postpronotum pilose. Anepisternum with bare part limited to ventral half, or entirely pilose. Antenna longer than distance between antennal fossa and anterior oral margin. Basoflagellomere shorter than or as long as scape. Tergite 1 short: length/width ratio 1:25 or less.
All included species (except the ones here described) were originally described in the genus Microdon. However, the morphology of Metadon is distinct. Characters that separate these taxa in all examined species (except Metadon bifasciatus, see below) are: anepisternum (almost) entirely pilose; phallus projecting not or only little beyond apex of hypandrium; aedegus furcate in apical half. Additional characters for distinguishing Metadon from Microdon (that may not work for all species) are: katepimeron more or less flat, with wrinkled texture; katatergum with oblique rows of microtrichia. In general, the abdomen of Metadon species is more elongate than that of Microdon species.
The East Palaearctic species Metadon bifasciatus Matsumura, 1916 is aberrant in certain characters. In this species the bare part of the anepisternum reaches up to about half the height of the sclerite. In addition, the genitalia are aberrant as the ventral aedeagal process is much longer than the dorsal process (Fig. 172), a character not known from any other species of Microdontinae. Nevertheless, this species is placed in Metadon because of the elongate abdomen and the oblique rows of microtrichia on the katatergum. This is supported by the results of Reemer and Ståhls (in press). As the Chinese species Microdon brunneipennis Huo, Ren & Zheng, 2007 and Microdon pingliensis Huo, Ren & Zheng, 2007 and Microdon spuribifasciatus Huo, Ren & Zheng, 2007 are similar to Microdon bifasciatus, the characters as mentioned may also be valid for those species.
Metadon is erected as a new genus distinct from Microdon in order to facilitate distinction between these apparently monophyletic groups. Results of phylogenetic analyses by Reemer and Ståhls (in press) support this decision.
Described species: 42. About half of the species (22) are described from the Oriental region. Several undescribed species from this region were seen by the first author in different collections. From the Afrotropical region, 14 species are described, remarkably none of which is from Madagascar. Four species are known from the Palaearctic region. These seem to form a closely related species group, all related to Microdon bifasciatus, restricted to eastern China, Korea and Japan. Two species are known from the Aru Islands off the southwest coast of New Guinea (these were collected by Alfred Russel Wallace in 1857, to be described by Walker 1858). These are the only known records of this group from the Australian region.
Microdon Meigen
Figs 176–240
Microdon Meigen, 1803: 275. Type species: Musca mutabilis Linnaeus, 1758: 592, by monotypy.
Aphritis Latreille, 1804: 193. Type species: Aphritis auropupescens Latreille, 1805, by subsequent monotypy. See Cheng and Thompson (2008) for synonymy.
Colacis Gistel, 1848: x. New name for Microdon Meigen. See Cheng and Thompson (2008) for synonymy.
Scutelligera Spix, 1824: 148. Type species: Scutelligera ammerlandia Spix, 1824: 124, by monotypy. See Cheng and Thompson (2008) for synonymy.
Parmula Heyden, 1825: 589. Type species: Parmula cocciformis Heyden, 1825: 589, by monotypy. See Cheng and Thompson (2008) for synonymy.
Scutigerella Haas, 1924: 148. Misspelling of Scutelligera Spix, 1824. See Cheng and Thompson (2008) for synonymy.
Subgenera (see separate accounts below)
Chymophila Macquart, 1834
Dimeraspis Newman, 1838 (= Mesophila Walker, 1849, syn. n.)
Megodon Keiser, 1971
Microdon s.s.
Myiacerapis Hull, 1949
Syrphipogon Hull, 1937
Species groups (see under Microdon s.l., after subgenera)
craigheadii-group
erythros-group
mirabilis-group
tarsalis-group
virgo-group
Unplaced species (see under Microdon s.l., after subgenera)
Microdon amabilis Ferguson, 1926
Microdon carbonarius Brunetti, 1923
Microdon macquariensis Ferguson, 1926
Microdon nigromarginalis Curran & Bryan, 1926
Microdon pictipennis (Macquart, 1850)
Microdon rieki Paramonov, 1957
Microdon trimacula Curran, 1928
Microdon unicolor Brunetti, 1915
Microdon waterhousei Ferguson, 1926
Body length: 10–16 mm. Broadly built flies with oval to round abdomen and long antennae. Head about as wide as to slightly narrower than thorax. Face convex in profile; slightly narrower to slightly wider than an eye. Lateral oral margins produced. Vertex flat. Occiput ventrally narrow, dorsally widened. Eye bare or very short pilose. Eye margins in male converging at level of frons, with mutual distance 1-3 times as large as width of antennal fossa. Antennal fossa about as wide as high. Antenna longer than distance between antennal fossa and anterior oral margin; basoflagellomere shorter than scape; bare. Postpronotum pilose. Scutellum trapezoid; with calcars. Propleuron pilose. Anepisternum with sulcus; pilose anterodorsally and posteriorly, extensively bare ventrally and medially. Anepimeron entirely pilose. Katepimeron convex; smooth; bare. Katatergum uniformly microtrichose. Wing: vein R4+5 with posterior appendix; vein M1 with outward angle, often with outward appendix, anteriorly recurrent; postero-apical corner of cell r4+5 widely rounded, with or without appendix; crossvein r-m located between basal 1/5 and 1/3 of cell dm. Abdomen oval, 1-1.5 times as long as wide. Tergites 3 and 4 fused. Sternite 1 pilose. Male genitalia: phallus projecting far beyond apex of hypandrium, bent dorsad, furcate basally, with both processes equally long and very slender; epandrium with ventrolateral ridge; surstylus with two wide lobes; subepandrial sclerite with elongate anterior projection projecting well beyond surstylus in lateral view.
Vein R4+5 with posterior appendix. Abdomen oval. Vein M1 of characteristic shape: with outward angle, usually with small outward appendix, anteriorly recurrent (Fig. 177). In addition to this character, this subgenus also differs from Microdon s.s. in the aedeagal processes being longer and more slender, and in the subepandrial sclerite projecting anteriorly well beyond the surstylus in lateral view (Fig 178–180).
Species of this group are similar in overall habitus to Microdon s.s. Many species have metallic colours, but some are dull black or have a ‘tiger-striped’ abdomen. Previously, this group was considered to be exclusively Neotropical (Cheng and Thompson 2008). However, several Oriental and one Japanese species are very similar to the Neotropical species in both external characters and morphology of the male genitalia.
Described species: 34. Neotropical (25 species), Oriental (7 species), Nearctic (1 species) and Eastern Palaearctic (1 species from southern Japan).
Body length: 8–12 mm. Broadly built flies with oval to round abdomen and long antennae. Head narrower than to about as wide as thorax. Face convex in profile; narrower to wider than an eye. Lateral oral margins not produced. Vertex flat. Occiput ventrally narrow, dorsally widened or narrow (only in Microdon abditus Thompson, 1981). Eye bare. Eye margins in male converging at level of frons, sometimes only weakly so (Microdon adventitius Thompson, 1981, Microdon fuscipennis (Macquart, 1834)) with mutual distance 2-5 times as large as width of antennal fossa. Antennal fossa about as wide as high. Antenna longer than or as long as distance between antennal fossa and anterior oral margin; basoflagellomere shorter to longer than scape; bare. Postpronotum pilose. Scutellum semicircular to trapezoid; without calcars, but large and blunt calcars may seem to be present due to strong apicomedian sulcus. Propleuron bare. Anepisternum without sulcus (or only a very weak one dorsally); pilose dorsally, extensively bare on slightly more or slighly less than ventral half. Anepimeron entirely pilose. Katepimeron more or less convex; smooth or with wrinkled texture (Microdon fuscipennis); bare. Katatergum uniformly microtrichose. Wing: vein R4+5 with posterior appendix; vein M1 more or less straight, perpendicular to vein R4+5, slightly recurrent; postero-apical corner of cell r4+5 rectangular, with appendix; crossvein r-m located between basal 1/7 and 1/4 of cell dm. Abdomen oval, 1-1.5 times as long as wide. Tergites 3 and 4 fused. Sternite 1 pilose or bare. Male genitalia: phallus projecting little beyond apex of hypandrium, bent dorsad, furcate apically, with both processes equally long; epandrium with ventrolateral ridge; surstylus with wide basal lobe and narrow posterior lobe.
Difficult to diagnose, because included species vary strongly in several key characters. See key and discussion.
This group was erected for the Nearctic Dimeraspis podagra Newman, 1838, a subjective synonym of Mulio globosus Fabricius, 1805 (Thompson 1981b). This species differs from Microdon s.s. in the unsulcate anepisternum, the bare propleuron, the rectangular postero-apical corner of cell r4+5, and the male genitalia: phallus apically furcate, hypandrium with bulb-like base. Some other Nearctic (and one Cuban) species are very similar in morphology of the male genitalia: Microdon abditus Thompson, 1981, Microdon adventitius Thompson, 1981, Microdon fuscipennis (Macquart, 1834), Microdon marmoratus Bigot, 1883, and Microdon remotus Knab, 1917. Thompson (1981b) also regarded these species as related, with the ‘globosus complex’ (Microdon abditus, Microdon globosus, Microdon marmoratus) as sister to the fuscipennis-group (Microdon adventitius, Microdon fuscipennis, Microdon remotus). These species are also similar in their overall brownish colouration and in the wing venation. The morphological similarities are here taken as a reason to include all species in Dimeraspis. Because of similarities in male genitalia this group might tentatively be considered related to Archimicrodon, Menidon or Serichlamys. However, because of considerable uncertainty, the group is here treated as a subgenus of Microdon.
Mesophila Walker, 1849 was erected for Ceratophya fuscipennis Macquart. As this species is here included in the older genus group Dimeraspis, Mesophila becomes a junior synonym of Dimeraspis.
Described species: 5. Nearctic (4 species) and West Indian (1 species from Cuba).
Body length: 8–13 mm. Broadly built flies with oval abdomen and long antennae. Head about as wide as thorax. Face convex in profile; narrower than an eye. Lateral oral margins slightly produced. Vertex flat. Occiput narrow and parallel-sided over entire length. Eye bare. Eye margins in male converging at level of frons, with mutual distance about equal to width of antennal fossa. Antennal fossa about as wide as high. Antenna longer than distance between antennal fossa and anterior oral margin; basoflagellomere shorter than to as long as scape; bare. Postpronotum pilose. Scutellum trapezoid; with strongly developed calcars. Anepisternum weakly sulcate; pilose anterodorsally and along posterior margin, widely bare in between. Anepimeron entirely pilose. Katepimeron convex; smooth; bare. Wing: vein R4+5 with posterior appendix; vein M1 more or less straight, perpendicular to vein R4+5; postero-apical corner of cell r4+5 angular to weakly rounded, with or without appendix; crossvein r-m located around basal 1/6 of cell dm. Abdomen oval, around 1.5 times as long as wide. Tergites 3 and 4 fused. Sternite 1 pilose or bare. Male genitalia: phallus furcate near base, with processes equally long and projecting well beyond apex of hypandrium; epandrium with ventrolateral ridge; surstylus unfurcate, elongate, curved dorsad.
Vein R4+5 with posterior appendix. Postpronotum pilose. Abdomen oval. Anepisternum widely bare medially. Propleuron bare. Postero-apical corner of cell r4+5 more or less rectangular. Antenna longer than distance between antennal fossa and anterior oral margin. Occiput narrow and parallel-sided over entire length (not widened dorsally). First tarsomere of hind leg dorsally with wide longitudinal groove.
Keiser (1971) erected this genus to include a species with very large, cone-shaped scutellar calcars. Cheng and Thompson (2008) did not study this species and refrained from commenting on the status of the group. The first author was able to study the holotype of Megodon stuckenbergi Keiser, 1971, as well as some additional material.
Megodon is very similar in external morphology to Microdon. Their genitalia also share important characters, like the deeply furcate phallus, the long aedeagal processes and the presence of a ventrolateral ridge on the epandrium. There are also differences, most notably the entirely narrow and parallel-sided occiput, and the dorsal, longitudinal groove on the first tarsomere of the hind leg. The shared characters are here considered more important than the differences. Because of these considerations, combined with the phylogenetic results of Reemer and Ståhls (in press), Megodon is here treated as a subgenus of Microdon.
Microdon planitarsus Keiser, 1971 is here also assigned to Megodon, because it agrees with the diagnostic characters as described above, and its male genitalia are very similar to those of Microdon stuckenbergi (Figs 193, 195). In Microdon planitarsis, the scutellar calcars are not as large and cone-shaped as in Microdon stuckenbergi. This indicates that the size and shape of these calcars should not be regarded as group-defining.
Described species: 2. Madagascar. One undescribed species from Madagascar is known to the first author.
Body length: 7–14 mm. Broadly built flies with oval abdomen and long antennae. Head narrower to slightly wider than thorax. Face convex in profile; slightly narrower to wider than an eye. Lateral oral margins not or weakly produced. Vertex flat. Occiput ventrally narrow to wide, dorsally widened. Eye bare. Eye margins in male converging at level of frons, with mutual distance 2-4 times as large as width of antennal fossa. Antennal fossa about as wide as high. Antenna longer than distance between antennal fossa and anterior oral margin; basoflagellomere shorter to longer than scape; bare. Postpronotum pilose. Scutellum semicircular to trapezoid; with or without calcars. Propleuron pilose. Anepisternum sulcate; pilose anterodorsally and posteriorly, widely bare ventrally and medially. Anepimeron entirely pilose. Katepimeron convex; smooth; bare. Katatergum uniformly microtrichose. Wing: vein R4+5 with posterior appendix; vein M1 more or less straight, perpendicular to vein R4+5, sometimes with slight inward angle in anterior 1/3; postero-apical corner of cell r4+5 rounded, with or without appendix; crossvein r-m located between basal 1/6 and 1/4 of cell dm. Abdomen oval, 1-1.5 times as long as wide. Tergites 3 and 4 fused. Sternite 1 pilose. Male genitalia: phallus projecting clearly beyond apex of hypandrium, bent dorsad, furcate close to base, with both processes about equally long or dorsal process longer than ventral process; epandrium with ventrolateral ridge; surstylus with two short, wide lobes.
Vein R4+5 with posterior appendix. Postpronotum pilose. Abdomen oval. Anepisternum extensively bare ventrally and medially. Postero-apical corner of cell r4+5 rounded. Katepimeron convex, without microtrichia. Apical crossvein M1 without outward angle. Lateral oral margins not or weakly produced.
As Cheng and Thompson (2008) wrote, this genus has remained “somewhat a catch all for various unrelated species not placed in other genera”. Many species previously placed inMicrodon are transferred to other genera in the present paper, e.g. Archimicrodon, Metadon and Peradon. These classificatory changes are supported by the results of the phylogenetic analysis of combined molecular and morphological characters by Reemer and Ståhls (in press). The analysis of only morphological characters by Reemer and Ståhls (in press) included many additional species which do not obviously belong to any of the previously recognized genus groups, nor to the genera erected in the present paper. The phylogenetic results offer little or no clues as to their taxonomic affinities. As most of these species were originally described in Microdon, and were subsequently maintained in that genus, the pragmatic solution is here chosen to keep these taxa in Microdon s.l. (see below). This category should not be confused with the supposedly monophyletic Microdon s.s. as defined above, as Microdon s.l. is probably not monophyletic. For some of these taxa, genus group names are available, which are here treated as subgenera (see separate accounts). The other taxa are here placed in species groups or left unplaced. These taxa are discussed below.
Unlike the other species groups discussed below, the virgo species group is considered to belong within Microdon s.s.
Described species: 62. Occurs in Nearctic (13 species), Neotropical (14), Oriental (9) and Palaearctic (26) regions.
Body length: 12 mm. Broadly built flies with bee-like pilosity and long antennae. Head wider than thorax. Face convex; wider than an eye. Lateral oral margins not produced. Vertex flat. Occiput ventrally narrow, dorsally widened. Eye bare or very short and sparsely pilose. Eye margins in male hardly converging at level of frons, with mutual distance about 5 times as large as width of antennal fossa. Antennal fossa about as wide as high. Antenna longer than distance between antennal fossa and anterior oral margin; basoflagellomere longer than scape; bare. Postpronotum pilose. Scutellum semicircular; without calcars. Anepisternum weakly sulcate; pilose anteriorly and posteriorly, widely bare in between. Anepimeron entirely pilose. Katepimeron convex; with wrinkled texture; bare. Wing: vein R4+5 with posterior appendix; vein M1 slightly recurrent, but more or less perpendicular to vein R4+5; postero-apical corner of cell r4+5 rounded, without appendix; crossvein r-m located around basal 1/4 of cell dm. Abdomen oval, about 1.5 times as long as wide. Tergites 3 and 4 fused. Sternite 1 pilose. Male genitalia: phallus furcate, with furcation point near base; epandrium with ventrolateral ridge; surstylus unfurcate.
Abdomen oval, about 1.5 times as long as wide. Vein R4+5 with posterior appendix. Postpronotum pilose. Proepimeron bare. Antenna longer than distance between antennal fossa and anterior oral margin. Basoflagellomere longer than scape. Anepisternum with bare ventromedian part extending to dorsal half. Sternite 1 pilose. Scutellum without calcars.
Myiacerapis was described as a subgenus of Microdon. In morphology it is quite similar to Microdon s.s. as defined in the present paper, also in the male genitalia (deeply furcate phallus with equally long processes, epandrium with ventrolateral ridge). However, unlike Microdon s.s. it has a bare proepimeron (pilose in Microdon s.s.) and a wrinkled texture of the katepimeron. Therefore, it is not placed in Microdon s.s. here, but in Microdon s.l, in awaitance of better understanding of its phylogenetic affinities.
Described species: 1. Africa (Uganda). An undescribed species is known from South Africa (coll. BMNH).
Body length: 25–28 mm. Very large flies with oval abdomen and long, colourful pilosity. Mimics of orchid bees of the genus Eulaema (Euglossidae). Head about as wide as thorax. Face more or less straight in profile; narrower than an eye; on ventral half with very long, thick and dense pile, resembling a beard (‘mystax’). Eye margins in male converging at level of frons, with mutual distance about twice as large as width of antennal fossa. Antennal fossa about as wide as high. Antenna longer than distance between antennal fossa and anterior oral margin; basoflagellomere shorter than scape, oval, about four times as long as wide, bare. Postpronotum bare. Scutellum trapezoid; with very large, cone-shaped calcars. Anepisternum sulcate; pilose anterodorsally and posteriorly, widely bare medially. Anepimeron entirely pilose. Katepimeron convex; smooth; bare. Wing: vein R4+5 with posterior appendix; vein M1 straight, perpendicular to vein R4+5; postero-apical corner of cell r4+5 widely rounded, without appendix; crossvein r-m located around basal 2/7 of cell dm. Abdomen oval, about 1.3 times as long as wide. Tergites 3 and 4 fused. Sternite 1 pilose. Male genitalia: phallus furcate, with furcation point near base, both processes about equally long, curved dorsad, projecting well beyond apex of hypandrium; epandrium without ventrolateral ridge; surstylus shallowly furcate, with two short and wide lobes.
Body length more than 20 mm. Face with very long, thick and dense pile, resembling a beard (‘mystax’).
Hull (1937b) erected Syrphipogon, and considered it related to Microdon. Steyskal (1953) referred to Hull’s description in his own description of an apparently very similar species (Microdon gaigei Steyskal, 1953), but he considered the differences with Microdon insufficient for generic status. In external characters and male genitalia Microdon and Syrphipogon are quite similar. For that reason, Syrphipogon is here still treated as a subgenus of Microdon.
The differences between the two species of Syrphipogon are not very convincing when comparing the description of Steyskal (1953), based on a female, with the holotype of Syrphipogon fucatissimus Hull, 1937, a male. The differences as noted by Steyskal (1953) may be due to sexual dimorphism, but in order to establish this, the type of Microdon gaigei needs to be examined.
Described species: 2. Neotropical. Only two specimens are known: one from Panama and one from “South America”.
craigheadii-group.
Only one species is included in this group: Microdon craigheadii Walton, 1912. This slender, metallic green Nearctic species is similar in habitus to Laetodon and many species of Microdon s.l. From these groups, Microdon craigheadii differs in the structure of the basal part of the phallus: the part of the phallus connecting the basal spherical part with the apical part is complexly curved (Fig. 232). This is a very unusual structure in Microdontinae, only found in this species and in Kryptopyga. In other genitalic structures (phallus deeply furcate, epandrium with ventrolateral ridge) as well in external morphology, Microdon craigheadii is very similar to Microdon s.s. Because of the peculiar morphology of the genitalia, the species is placed in a separate species group within Microdon s.l.
erythros-group.
In overall habitus and many external characters, the species of this group remind of both Microdon s.s. and Metadon. Placement in Microdon s.s. is contradicted by the phallus being furcate apically (Fig. 233), whereas placement in Metadon is contradicted by the extensively bare anepisternum. As the phylogenetic analysis of morphological characters (Reemer and Ståhls in press) provides no information on the affinities of Microdon erythros Bezzi, 1908, this species is placed in Microdon s.l., along with the similar Microdon luteiventris Bezzi, 1915.
mirabilis-group.
The species of this Neotropical group have contrasting yellow and black colour patterns on the wings, combined with remarkably long hind legs, evoking a resemblance to certain Pompilidae (Hymenoptera). Apart from this, they differ from Microdon s.s. in the bare propleuron and the aedeagal processes projecting hardly beyond the apex of the hypandrium.
Apart from Microdon mirabilis, this group includes Microdon bertonii Bezzi, 1910 (= Microdon arcuatus Curran, 1941, syn. n.) and Microdon iheringi Bezzi, 1910. The species seem to differ only in colouration of wings, legs and abdomen. However, quick glances in museum collections (e.g. USNM) suggest that intermediate specimens exist. This indicates that species taxonomy of this group needs further attention.
Bezzi (1910) wrote that he had two male specimens Microdon iheringi in his collection, which he both considered as ‘cotypes’. The collection of the MCSN (Milan) presently holds only one specimen (a male), which was examined by the first author. It is uncertain whether the other specimen still exists. In order to assure stability of this taxon, the specimen in the MCSN-collection is here designated as lectotype. Label information is as follows: label 1: “5695”; label 2: “S. Paulo / Brasile / 26.X.06 / Hering”; label 3: “iheringi”; label 4 (red): “LECTOTYPE / Microdon iheringi / Bezzi, 1910 / Des. M. Reemer 2009”.
tarsalis-group.
This group only includes the Afrotropical species Microdon tarsalis Hervé-Bazin, 1913 and its synonym Microdon bequaerti Curran, 1929 (syn. n.). In the phylogenetic analysis of morphological characters (Reemer and Ståhls in press) this species was recovered in the Microdon s.l. clade, but its exact relationship with the other groups in this clade were unresolved. This group differs from Microdon s.l. in e.g. the entirely narrow occiput, the short and characteristically shaped phallus with the dorsal process longer than the ventral process, and the absence of a ventrolateral ridge on the epandrium. Besides, there is a patch of pile with hook-shaped apexes on the hind basitarsus dorsally on its inner surface.
In overall habitus (including swollen hind basitarsus), Microdon tarsalis is remarkably similar to the Nearctic Microdon (Dimeraspis) abditus Thompson, 1981 but considering the differences in male genitalia this similarity is probably merely superficial. These differences are: phallus with dorsal process longer than ventral process (equally long in Microdon abditus), epandrium without ventrolateral ridge (with ventrolateral ridge in Microdon abditus), surstylus simple shaped, without distinct posterior process (with posterior process in Microdon abditus).
virgo-group.
This group consists of Neotropical metallic green, blue or bronze flies, sometimes partly reddish. It is differentiated from Microdon s.s. in the key by the bare propleuron and the strongly produced lateral oral margins, of which the anterolateral corners are distinctly angular (Fig. 229). The latter character is presented with some hesitation, as it is uncertain whether it works for all species. Possibly, certain species here placed in Microdon s.s. also belong in this group. Therefore, the virgo-group is here considered as a species group within Microdon s.s., instead of within Microdon s.l. As it is presently uncertain which species should be assigned to it, this group is not recognized in the species catalogue in this paper.
Unplaced species.
Several species of Microdon s.l. (see Appendix 2) do not fit into any of the groups described above. In the phylogenetic analyses of Reemer and Ståhls (in press), the following species belonging to this group were included: Microdon amabilis Ferguson, 1926, Microdon carbonarius Brunetti, 1923, Microdon nigromarginalis Curran & Bryan, 1926, Microdon pictipennis (Macquart, 1850), Microdon rieki Paramonov, 1957, Microdon trimacula Curran, 1928, Microdon tsara Keiser, 1971, Microdon waterhousei Ferguson, 1926. The results hardly offer solid clues as to their exact relationships with Microdon s.s. For examples of morphology of these species see Figs 230, 231, 237–240.
Diversity and distribution.
In total, 126 species (from all continents except Antarctica) are here classified under Microdon. Of these, 62 are placed in Microdon s.s., 46 in other subgenera, and 18 are not placed in subgenera (although some of them in species groups) but left in Microdon s.l.
http://species-id.net/wiki/Mixogaster
Figs 241–248Body length: 9–15 mm. Slender flies with constricted abdomen, wasp-like. Head wider than thorax. Face convex or almost straight in profile; about as wide as an eye or narrower. Lateral oral margins not produced. Vertex flat. Occiput narrow, except slightly widened dorsally. Eye bare. Eyes in male not or hardly converging at level of frons, with mutual distance 4 to 5 times as large as width of antennal fossa. Antennal fossa about as wide as high. Antenna longer or shorter than distance between antennal fossa and anterior oral margin; basoflagellomere shorter to longer than scape, oval; bare. Postpronotum pilose. Scutellum semicircular; without calcars. Anepisternum with weak sulcus; entirely bare or pilose anterodorsally, or pilose anterodorsally and along posterodorsal margin. Anepimeron entirely pilose or bare on ventral half. Katepimeron convex; bare. Wing vein R4+5 without posterior appendix. Vein M with small anterior appendix into cell r4+5. Vein M1 either straight or with anterior part directed outward, with one or two angles, whether or not with small inward appendix and /or small outward appendix. Postero-apical corner of cell r4+5 angular. Crossvein r-m located betwee basal 1/4 to 2/5 of cell dm. Abdomen constricted at base, with tergite 2 varying in length and width. Tergites 3 and 4 not fused. Male genitalia: phallus unfurcate, bent dorsad, with either lateral or dorsal combined with ventral lamellae, sometimes with apical spines; hypandrium with bulb-like base and apical part consisting of separate lobes, or hypandrium entirely consisting of two separate parts, which are not interconnected; epandrium without ventrolateral ridge; surstylus of varying shape.
Vein M with small anterior appendix into cell r4+5. Abdomen constricted. Metapleura connected, postmetacoxal bridge complete.
An important diagnostic character of Mixogaster, the anterior appendix of vein M, is also found in Spheginobaccha de Meijere, 1908 and certain specimens of Aristosyrphus primus. These taxa also share the character of the apical part of the hypandrium consisting of two separate lobes. See genus account of Aristosyrphus for discussion.
The morphology of the male genitalia is remarkably diverse in this genus, much more so than in other groups of Microdontinae (except perhaps Aristosyrphus / Eurypterosyrphus). Some species have characters not known from any other Microdontinae. Some examples are illustrated in Figs 245–248. In Mixogaster breviventris Kahl, 1897, the phallus has wide dorsal and ventral lamellae (Fig. 245). This type of genitalia is found in all Nearctic species, which also have a straight vein M1 in common. In Microdon thecla Hull, 1954 (Fig. 247), the hypandrium consists of two separate lobes, which are not interconnected ventrally to envelope the phallus, as is the case in all other studied Microdontinae. Besides, the subepandrial sclerite is strongly developed in this species, and produced well beyond the epandrium in lateral view. In an undescribed species (Fig. 248), the phallus is asymmetric in ventral view, with wide lateral lamellae, which are apically densely occupied with irregular spines. This is the only known case of asymmetric genitalia among Microdontinae. The spinose phallus is also a unique character.
The keys to the species by Hull (1954) and Carrera and Lenko (1958) (Brazilian species only) work reasonably well, but the existence of several undescribed species makes it necessary to check original descriptions or, preferably, type material in order to verify identifications. Considering the large interspecific variation in the male genitalia, these characters should be further explored in future (re)descriptions of species.
Described species: 21. Mainly Neotropical, with three species in the Nearctic. At least one Nearctic and several Neotropical species are undescribed.
http://species-id.net/wiki/Oligeriops
Figs 249–253Body length: 7–10 mm. Dark-coloured, stout-legged flies with oval abdomen and moderately long antennae. Head about as wide as thorax. Face convex; wider than an eye. Lateral oral margins produced. Vertex flat. Occiput wide over entire length, narrowest point halfway. Eye bare. Eye margins in male not converging at level of frons, with mutual distance around 4 times as large as width of antennal fossa. Antennal fossa about as wide as high. Antenna longer than distance between antennal fossa and anterior oral margin; basoflagellomere longer than scape; with dorsal margin curved dorsad, more or less sickle-shaped; bare. Postpronotum pilose. Scutellum semicircular; without calcars. Anepisternum weakly sulcate; pilose, with small bare part on ventral half. Anepimeron entirely pilose. Katepimeron convex; with wrinkled texture; bare. Wing: vein R4+5 with posterior appendix; vein M1 more or less straight, perpendicular to vein R4+5; postero-apical corner of cell r4+5 rectangular, with small appendix; crossvein r-m located between basal 1/6 of cell dm. Abdomen oval, about twice as long as wide. Tergites 3 and 4 fused. Sternite 1 pilose. Male genitalia: phallus not or little projecting beyond apex of hypandrium, slightly bent dorsad, shallowly furcate, with both processes about equally long; epandrium without ventrolateral ridge; surstylus unfurcate.
Vein R4+5 with posterior appendix. Postpronotum pilose. Abdomen oval. Anepisternum largely pilose, at most with small bare part on ventral half. Basoflagellomere sickle-shaped: dorsal margin curved upward.
Hull (1937a) described Oligeriops as a genus, with only Microdon chalybeus Ferguson, 1926 included, without indicating its diagnostic generic characters. Hull (1949) used the reduced size of the eyes (due to widened occiput and gena) and the sickle-shaped antenna as key characters. Thompson and Vockeroth (1989) list Oligeriops as synonym of Microdon. Cheng and Thompson (2008) express their doubts about ranking Oligeriops as a genus, while referring to the antennae of Australian Microdon species as illustrated in Ferguson (1926b). These illustrations show that other species originally described in Microdon also have a curved basoflagellomere, just like Microdon chalybeus Ferguson, 1926, but nevertheless these species were not included in Oligeriops by Hull (1937a, 1949). Cheng and Thompson (2008) state that ‘Whether these other species have reduced eyes remains to be seen!’. However, as Ferguson (1926a, b) already noticed, the four species he described are all ‘close’ and ‘very similar’. Examination of type specimens, additional material and original descriptions, has confirmed this, and has made clear that all five species presently included in Oligeriops have reduced eyes and sickle-shaped basoflagellomeres indeed. Based on these and other morphological similarities, there is no doubt that they are closely related.
Oligeriops does not fit into the concept of Microdon s.s. as defined in the present paper. In addition to the reduced size of the eye and the curved basoflagellomere, the following characters distinguish Oligeriops from Microdon: anepisternum almost entirely pilose, at most with small bare part ventrally; propleuron bare; postero-apical corner of cell r4+5 rectangular; phallus projecting little beyond apex of hypandrium, furcate near apex. Considering these characters in combination with the results of Reemer and Ståhls (in press), it is deemed not appropriate to include this taxon in Microdon.
Described species: 5. Australia (incl. Tasmania).
http://species-id.net/wiki/Omegasyrphus
Figs 254–256Body length: 7–9 mm. Small, dark flies with relatively short antennae and characteristically shaped abdomen. Head slightly wider than thorax. Face convex; about as wide as or narrower than an eye. Lateral oral margins hardly produced. Vertex flat or slightly produced, densely pilose. Occiput ventrally narrow, dorsally widened. Eye bare. Eye margins in male slightly converging at level of frons, with mutual distance 2.5-3 times width of antennal fossa. Antennal fossa about as wide as high. Antenna shorter than distance between antennal fossa and anterior oral margin; basoflagellomere as long as or longer than scape, oval; bare. Postpronotum pilose. Scutellum semicircular; with calcars. Anepisternum sulcate; entirely pilose. Anepimeron entirely pilose. Katepimeron moderately convex; with wrinkled texture; bare. Wing: vein R4+5 with posterior appendix; vein M1 perpendicular to vein R4+5; postero-apical corner of cell r4+5 rectangular to weakly rounded, with small appendix; crossvein r-m located between basal 1/6 to 1/5 of cell dm. Abdomen 2.5-3 times as long as wide; with characteristic shape: widest point about halfway tergite 2, which has strongly arcuate lateral margins and pair of depressed areas dorsally; tergites 3-4 narrower and almost parallel-sided. Tergites 3 and 4 fused. Sternite 1 pilose. Male genitalia: phallus furcate near apex, with dorsal process long and whip-like, ventral process very short; epandrium with ventrolateral ridge.
Vein R4+5 with posterior appendix. Antenna shorter than distance between antennal fossa and anterior oral margin. Tergite 2 with strongly arcuate lateral margins, tergites 3-4 narrower and almost parallel-sided. Sternite 2 and 3 separated by membrane that is much less wide than sternite 2.
This group was treated as a subgenus of Microdon by Thompson (1981b). Based on the phylogenetic evidence of Reemer and Ståhls (in press) this ranking cannot be maintained. Instead, Omegasyrphus is treated as a distinct genus. Thompson (1981b), who gives a key to the North American species, points out that species level taxonomy is necessary for this genus. This is still true.
Described species: 5. North and Central America, from South Dakota in the U.S.A. southward to Guatemala. The south border of this range is marked by Microdon brunnipennis Hull, 1944, which was described as a variety of Microdon baliopterus Loew, 1872 by Hull (1944b). The assignment of this taxon to Omegasyrphus is based only on this description, as the type has not been examined.
http://species-id.net/wiki/Paragodon
Figs 257–261Body length: 4–5 mm. Small flies with short antennae and oval abdomen. Head slightly wider than thorax. Face convex; narrower than an eye. Lateral oral margins not produced. Vertex flat. Occiput ventrally narrow, dorsally widened. Eye bare. Eye margins in male not converging at level of frons, with mutual distance about three times as large as width of antennal fossa. Antennal fossa about as wide as high. Antenna shorter than distance between antennal fossa and anterior oral margin; basoflagellomere longer than scape, oval, about 1.5 times as long as wide, bare. Postpronotum pilose. Scutellum semicircular; without calcars. Anepisternum convex; pilose anteriorly and posterodorsally, widely bare in between. Anepimeron bare or with a few thick, seta-like pile dorsally. Katepimeron convex; bare. Wing: vein R4+5 without posterior appendix; vein M1 straight, perpendicular to vein R4+5; postero-apical corner of cell r4+5 rectangular, with small appendix; crossvein r-m located very close to base of cell dm. Abdomen oval, about 1.5 times as long as wide. Tergites 3 and 4 fused. Sternite 1 bare. Male genitalia: phallus unfurcate, straight, projecting only little beyond apex of hypandrium; hypandrium with bulb-like base; epandrium without ventrolateral ridge; surstylus unfurcate.
Abdomen oval; yellow and black. Vein R4+5 without posterior appendix. Crossvein r-m almost at same level as base of cell dm. Antenna shorter than distance between antennal fossa and anterior oral margin. Postpronotum pilose.
When Thompson (1969) described this genus, he stated that it appeared to be the most primitive microdontine fly known. This was based on a number of presumed plesiomorphic characters: 1) unsclerotized ejaculatory apodeme and sac; 2) short antenna; 3) underdeveloped and bare metasternum; 4) lack of basal setal patches on hind femur; 5) lack of a spurious vein; 6) lack of appendix on vein R4+5; 7) presence of a double sustentacular apodeme; 8) unfurcate phallus. Now that a larger number of taxa of Microdontinae could be studied, all of these characters were also found in other taxa (Reemer and Ståhls in press), except for the unsclerotized ejaculatory apodeme. See also discussion under Surimyia.
Described species: 1. Central America (Mexico, Costa Rica and Panama).
http://species-id.net/wiki/Paramicrodon
Figs 262–268Body length: 4–11 mm. Small, slender flies with short antennae and more or less parallel-sided abdomen. Head slightly wider than thorax. Face convex; narrower than an eye. Lateral oral margins not produced. Vertex flat. Occiput ventrally narrow, dorsally strongly widened. Eye bare. Eye margins in male only slightly converging at level of frons, with mutual distance 1.5-2.5 times as large as width of antennal fossa. Antennal fossa about as wide as high. Antenna shorter than distance between antennal fossa and anterior oral margin; basoflagellomere longer than scape, oval, about 1.5 times as long as wide, bare. Postpronotum pilose. Scutellum semicircular; without calcars. Anepisternum convex; pilose anteriorly and posteriorly, widely bare in between. Anepimeron entirely pilose. Katepimeron convex; bare. Wing: vein R4+5 without posterior appendix; vein M1 straight, perpendicular to vein R4+5; postero-apical corner of cell r4+5 rectangular, with small appendix; crossvein r-m located within basal 1/10 of cell dm. Abdomen elongate: more or less parallel-sided, may be subtly constricted at tergite 3 (male), or slightly oval (female); 2.5–4 times as long as wide. Tergites 3 and 4 fused (but distinct suture visible). Sternite 1 bare or pilose. Sternites 3-4 strongly narrowed; narrower than sternite 2, with wide membraneous parts laterally. Male genitalia: phallus furcate near apex, slightly bent dorsad, projecting well beyond apex of hypandrium; hypandrium with apical part consisting of two separate lobes; epandrium without ventrolateral ridge; surstylus of varying shape.
Vein R4+5 without posterior appendix. Postpronotum pilose. Antenna shorter than distance between antennal fossa and anterior oral margin. Vein M1 straight, not parallel to wing margin, perpendicular to both vein R4+5 and M. Mesonotum with transverse suture incomplete. Sternites 3-4 strongly narrowed; narrower than sternite 2, with wide membraneous parts laterally.
The synonymy of Syrphinella Hervé-Bazin, 1926 with Paramicrodon was suspected by Hull (1937a) and stated explicitly by Hull (1949). This subjective synonymy is here confirmed, based on examination of the type specimen of the type species. Myxogasteroides Shiraki, 1930 was treated as a synonym of Paramicrodon by Hull (1949) and Cheng and Thompson (2008), a synonymy followed here based on the description of the type species. The synonymy of Nannomyrmecomyia Hull, 1945 and Paramicrodon was stated by Thompson (1969, 1981a) and is also confirmed here based on examination of the type specimens.
Described species: 8. The range of this genus is interestingly disjunct, with six species from the Oriental Region (Thailand to Moluccas), one from New Guinea and two from the Neotropical region. At least one additional species occurs in the Neotropical region (unpublished observations by the first author), but more species-level work is needed to sort this out.
http://species-id.net/wiki/Paramixogaster
Figs 269–284Body length: 5–13 mm. Slender flies with constricted abdomen and long antennae, usually with black and yellow colour pattern, wasp mimics. Head wider than thorax. Face convex in profile; narrower than to wider than an eye. Lateral oral margins not produced. Vertex flat to strongly swollen. Occiput ventrally narrow, dorsally widened. Antennal fossa about as wide as high. Eye bare. Eye margins in male parallel, not converging at level of frons. Antenna longer than distance between antennal fossa and anterior oral margin. Basoflagellomere usually much longer than scape, except shorter in Paramixogaster illucens (Bezzi) and Paramixogaster luxor Curran (see Discussion); bare. Postpronotum bare. Mesoscutum with transverse suture usually incomplete, except complete in Paramixogaster contractus, Paramixogaster conveniens and Paramixogaster omeanus (see Discussion). Scutellum semicircular; without or with small calcars. Anepisternum convex or sulcate; entirely pilose or partly bare on ventral half. Anepimeron entirely pilose. Katepimeron convex; bare. Wing: vein R4+5 with or without posterior appendix; vein M1 perpendicular to vein R4+5 and vein M; postero-apical corner of cell r4+5 rectangular to somewhat acute, with small appendix; crossvein r-m located within basal 1/4 of cell dm. Abdomen elongate, at least 3 times as long as wide; constricted, with narrowest point at tergite 2 and widest point at tergite 3 or 4. Tergites 3 and 4 fused. Male genitalia: phallus furcate, with furcation point in distal 1/3; epandrium without ventrolateral ridge; surstylus weakly furcate, only in Paramixogaster luxor consisting of three distinct branches.
Postpronotum bare. Basoflagellomere at least three times as long as wide. Posterio-apical corner of wing cell r4+5 rectangular or somewhat acute. Abdomen usually constricted (i.e. with narrowest point at tergite 2 and widest point at tergite 3 or 4); if not, then the following three characters apply: 1) basoflagellomere 2-4 times as long as scape, 2) tergite 2 less than half as long as tergites 3 and 4 together, 3) face smooth medially (without vitta of transversely wrinkled texture).
Cheng and Thompson (2008) regarded Paramixogasteroides Shiraki, 1930 and Tanaopicera Hull, 1945 as subjective synonyms of Paramixogaster. Examination of the type species of Tanaopicera, Ceratophya variegata Walker, 1852, confirmed this opinion with regard to Tanaopicera. One of the characters Hull (1945) used to characterize Tanaopicera was ‘the high, greatly developed vertex’. However, the vertex in the holotype of Ceratophya variegata is neither high nor greatly developed. This species is very similar to other Paramixogaster-species in all important characters. The type species of Paramixogasteroides, Myxogaster variegata Sack, was not examined, but its description by Sack (1922) is clear enough to include this taxon in Paramixogaster.
Morphological variation among the species presently included in Paramixogaster is large. Although most species have a constricted abdomen in dorsal view, this is not the case in the African taxa Paramixogaster acantholepidis (Speiser, 1913) and Paramixogaster crematogastri (Speiser, 1913), and the Australian species Paramixogaster praetermissus (Ferguson, 1926). However, tergite 2 is dorsoventrally flattened in these species, so in lateral view their abdomen appears constricted. In all other important characters of external morphology and male genitalia these taxa belong in Paramixogaster, as corroborated by the results of the phylogenetic analysis based on morphology (Reemer and Ståhls in press).
Paramixogaster illucens (Bezzi, 1915) and Paramixogaster luxor (Curran, 1931) are the only species included in this genus in which the basoflagellomere is shorter than the scape. In Paramixogaster luxor, the shape of the surstylus also differs from the other species, as it consists of three separate branches (Fig. 282). Nevertheless, both species are included in Paramixogaster because agree with the diagnosis.
Paramixogaster contractus (Brunetti, 1923), Paramixogaster conveniens (Brunetti, 1923) and Paramixogaster omeanus (Paramonov, 1957) are aberrant from all other known species of Paramixogaster in their complete transverse suture. This character is also found in Indascia, which includes species which look superficially similar to these Paramixogaster-species. However, these species are here assigned to Paramixogaster, based on the phylogenetic analysis of their morphology (Reemer and Ståhls in press). In addition, they possess a diagnostic character for Paramixogaster: the bare postpronotum. The first two species, Paramixogaster contractus and Paramixogaster conveniens, differ from all other studied species of Microdontinae in the presence of pile on the metaepisternum. It will be interesting to re-evaluate their taxonomic affinities when additional material becomes available. At present, the species are only known from the holotypes, which both are females, so no characters of male genitalia or DNA could be analyzed.
As a consequence of transferring some species from other genera to Paramixogaster, replacement names had to be chosen for two species. Examination of the type of Microdon vespiformis de Meijere, 1908 made clear that this is a species of Paramixogaster. As Mixogaster vespiformis Brunetti, 1913 was later designated as the type species of Paramixogaster, these two names are now secondary homonyms. For the junior name, vespiformis Brunetti, the nomen novum Paramixogaster brunettii is proposed here. The other new name introduced here is Paramixogaster sacki for Paramixogasteroides variegata Sack, 1922, which is a junior secondary homonym of Ceratophya variegata Walker, 1852.
Described species: 26. Afrotropical (5 species), Oriental (12) and Australian region (9). Several additional species, from all three regions, await description.
http://species-id.net/wiki/Parocyptamus
Figs 285–290Body length: 11–15 mm. Slender flies with elongate, tapering abdomen and long antennae, black with metallic hues, wings infuscated. Head about as wide as thorax. Face approximately straight in profile, except for slight bulge below antenna; narrower than eye. Lateral oral margins strongly produced. Vertex flat. Occiput ventrally narrow, dorsally slightly widened. Antennal fossa about as wide as high. Eye bare. Eye margins in male parallel, not converging at level of frons, mutual distance about three times width of antennal fossa. Antennal fossa about as wide as high. Antenna longer than distance between antennal fossa and anterior oral margin. Basoflagellomere shorter than scape; oval; bare. Postpronotum pilose. Scutellum semicircular; without calcars. Anepisternum deeply sulcate; almost entirely pilose, except bare on small part ventrally. Anepimeron entirely pilose. Katepimeron convex; bare. Wing: vein R4+5 with posterior appendix; vein M1 perpendicular to vein R4+5; postero-apical corner of cell r4+5 widely rounded; crossvein r-m located around basal 1/6 of cell dm. Abdomen elongate, more than 3 times as long as wide; in male gradually tapering from anterior half of tergite 2 to apex; in female slightly constricted between tergites 3 and 4. Tergites 3 and 4 fused. Male genitalia: phallus furcate basally, with dorsal process much longer than ventral one, projecting far beyond apex of hypandrium; epandrium with ventrolateral ridge; surstylus weakly furcate, divided into two short lobes.
Diagnosis. Vein R4+5 with posterior appendix. Postpronotum pilose. Anepisternum almost entirely pilose, except bare on small ventral part. Basoflagellomere shorter than scape. Abdomen at least three times as long as wide. Tergite 2 with pair of depressed areas (Fig. 287).
When Shiraki (1930) described Parocyptamus, this genus was diagnosed in a key by the following two characters: abdomen narrow and elongate, frons with antennal prominence (‘Fühlervorsprung’). The latter character is of limited use, as the frons is more or less extended above the antennae in many other taxa of Microdontinae. Hull (1937a) did not state which characters he considered diagnostic in his description of Stenomicrodon. Judging from his remarks in Hull (1949), the shape of the abdomen and the presence of a patch of short, spinose setae at the base of the front and mid femora were considered important characters. Although the anterobasal patches of setae are well-developed, such patches are also found in several other taxa of Microdontinae. Perhaps the spines are somewhat stronger developed than in most taxa, but it is hard to describe this as a discrete character state. Therefore, this character is not used in the present key and Diagnosis.
The abdomen is constricted (slightly) only in the female, not in the male, as might be erroneously concluded from the key of Cheng and Thompson (2008).
The synonymy of Stenomicrodon with Parocyptamus was already established by Hull (1949). Examination of the involved type specimens by the first author has confirmed this (subjective) synonymy. The type species of both genus group names are here also considered as synonyms (Parocyptamus sonamii Shiraki, 1930 = Stenomicrodon purpureus Hull, 1937 syn. n.). Microdon stenogaster Curran, 1931 also belongs to this genus, as it is almost identical to the type species in colouration, external morphology and male genitalia. Closer examination of available specimens, also from Sumatra and Thailand, is necessary to resolve species level taxonomy.
Shiraki (1930) based his description of Parocyptamus sonamii on three males. Two of these syntypes are kept in the NIAS collection. The third male (from Sokotsu) is apparently lost. Label information is as follows. Syntype 1: label 1: “Formosa, Shinchiku, -18. VII 1-30. J. Sonan, K. Miyake”; label 2: “Parocyptamus sonamii”; label 3 (round, red-bordered): “Type”. Syntype 2: label 1: “CIHpOn, 17.VII.1922, M. Yoshino”; label 2 (round, red-bordered): “Type”. The date on the label of syntype 1 is a bit cryptic (“-18. VII 1-30”). It is unlikely to assume the specimen has been collected in July 1930, because Shiraki’s work was published on the 30th of January 1930. It seems more plausible that the date was 1-30 July 1918. Shiraki (1930) only mentions the month (VII).
Described species: 2. Oriental: known from Taiwan, Thailand, Sumatra and Borneo.
urn:lsid:zoobank.org:act:4FB70E62-18D6-4B74-B96D-296B925BACBB
http://species-id.net/wiki/Peradon
Figs 291–298Body length: 6–18 mm. Slender to moderately broadly built flies with oval or basally constricted abdomen and long antennae. Head wider than thorax. Face straight to slightly convex or slightly concave in dorsal half; narrower to wider than an eye; medially with vitta of transversely wrinkled texture (except in some smaller species of the flavofascium-group); gena distinctly ventrally produced. Lateral oral margins produced. Vertex flat. Occiput ventrally narrow, dorsally widened. Eye bare. Eye margins in male converging at level of frons, with mutual distance 1.5-4 times as large as width of antennal fossa. Antennal fossa about as wide as high. Antenna longer than distance between antennal fossa and anterior oral margin; basoflagellomere shorter to longer than scape; bare. Postpronotum pilose or bare. Scutellum semicircular; with calcars. Anepisternum sulcate; pilose anterodorsally and posteriorly, widely bare in between. Anepimeron entirely pilose. Katepimeron flat; with wrinkled texture; bare. Wing: vein R4+5 with posterior appendix; vein M1 more or less straight, perpendicular to vein R4+5; postero-apical corner of cell r4+5 widely rounded, without appendix; crossvein r-m located between basal 1/6 and 1/3 of cell dm. Abdomen oval or basally constricted, 2-4 times as long as wide. Tergites 3 and 4 fused. Sternite 1 bare. Male genitalia: phallus not or little projecting beyond apex of hypandrium, slightly bent dorsad, shallowly furcate, with both processes about equally long and with their apexes wide at the furcation point but pointed apically; epandrium without ventrolateral ridge; surstylus unfurcate.
Vein R4+5 with posterior appendix. Postero-apical corner of cell r4+5 widely rounded. Katepimeron flat, with wrinkled texture, bare. Face in profile slightly convex, straight or slightly concave, but never bulged in ventral half. Vertex flat.
Three species groups are recognized here. These groups may not be monophyletic, but they may be useful for purposes of species identification. They are diagnosed as follows:
bidens-group: Abdomen oval or parallel-sided. Tergites without golden pile. Basoflagellomere less than twice as long as scape.
flavofascium-group: Abdomen oval. Tergite 4 often with golden or silver pile. If not, then basoflagellomere more than twice as long as scape.
trivittatus-group: Abdomen constricted basally.
The species here assigned to this genus (see Appendix 2) used to be placed in Microdon (e.g. Thompson et al. 1976), but the results of the phylogenetic analyses by Reemer and Ståhls (in press) indicate that they do not belong there. Based on external characters this group is difficult to diagnose, but usually the species can be distinguished at a glance from those of Microdon because of their more or less elongate (sometimes constricted) abdomen. In addition, morphology of the phallus is very constant (differences with Microdon in parentheses): projecting not or little beyond apex of hypandrium (far beyond apex of hypandrium), slightly bent dorsad (strongly bent), shallowly furcate (deeply furcate), with both processes about equally long and with their apexes wide at the furcation point but pointed apically.
Only one species here included in Peradon was previously not classified in Microdon: Ubristes chrysopygus Giglio-Tos, 1892.
Described species: 24. Neotropical. Several undescribed species are known to the first author.
The generic name is a combination of the Greek words peras (west) and odon, with the latter used as a suffix derived from Microdon. The prefix pera- is used to emphasize that this genus is restricted in its distribution to the western hemisphere.
urn:lsid:zoobank.org:act:70D34EA7-E2E7-4868-AE3E-AC5E74A3DDFD
http://species-id.net/wiki/Piruwa
Figs 299–306Body length: 4 mm. Small, slender flies with short antennae and constricted abdomen. Head slightly wider than thorax. Face convex; narrower than an eye. Lateral oral margins not produced. Vertex flat. Occiput narrow over entire length. Eye bare. Eye margins in male not converging at level of frons, with mutual distance 3 times as large as width of antennal fossa. Antennal fossa about as wide as high. Antenna shorter than distance between antennal fossa and anterior oral margin; basoflagellomere longer than scape, oval, about twice as long as wide, bare. Postpronotum bare. Scutellum semicircular; without calcars; with long bristly pile along margin, clearly longer and thicker than pile on rest of scutellum. Anepisternum convex; pilose anterodorsally and along posterodorsal margin. Anepimeron pilose along dorsal margin. Katepimeron convex; bare. Wing: vein R4+5 without posterior appendix; vein M1 straight, perpendicular to vein R4+5; postero-apical corner of cell r4+5 rectangular, with small appendix; crossvein r-m located within basal 1/10 of cell dm. Abdomen constricted, narrowest at transition between tergites 1 and 2, widest at tergite 4; about 2.5 times as long as wide. Tergites 3 and 4 fused, no suture visible. Sternite 1 bare. Male genitalia: phallus furcate near apex, slightly bent dorsad, projecting only a little beyond apex of hypandrium; hypandrium with bulb-like base, with apical part entire, not consisting of two separate lobes; epandrium without ventrolateral ridge; surstylus consisting of two lobes, with basal lobe angular, apical lobe rounded.
Vein R4+5 without posterior appendix. Antenna shorter than distance between antennal fossa and anterior oral margin. Postpronotum bare. Abdomen constricted.
Although there is a superficial similarity in habitus to Paramicrodon (small, slender, short antennae, vein R4+5 without posterior appendix), Piruwa differs from that genus in the following important characters: occiput narrow over entire length; postpronotum bare; scutellum with long bristly pile along margin; anepimeron pilose only along dorsal margin; sternites 3-4 about as wide as sternite 2; hypandrium with apical part not consisting of two separate lobes. Considering these differences, a close relationship between these taxa seems unlikely. Because of these differences and the uncertainy of taxonomic affinities, this distinct taxon is given generic rank.
Described species: 1. Neotropical. Only known from Peru.
The name Piruwa is derived from Piruw, the word for Peru in Quechuan, a native Andean-Ecuadorian language. It is to be treated as feminine.
http://species-id.net/wiki/Pseudomicrodon
Figs 307–320Body length: 7–9 mm. Slender flies with long antennae and petiolate abdomen. Head a little wider than thorax. Face more convex or straight in profile; narrower than to as wide as an eye. Lateral oral margins weakly produced. Vertex convex and shining; sparsely pilose, sometimes bare on anterior half. Occiput ventrally narrow, dorsally strongly widened. Eye bare or with very short and sparse pile. Eye margins in male converging at level of frons, with mutual distance 1-2 times width of antennal fossa. Antennal fossa about as wide as high to 1.5 times as wide as high. Antenna longer than distance between antennal fossa and anterior oral margin; basoflagellomere shorter to longer than scape, oval; bare. Postpronotum pilose. Scutellum semicircular; with or without calcars. Anepisternum sulcate; entirely pilose or widely bare medially. Anepimeron entirely pilose. Katepimeron flat to convex; usually with wrinkled texture; bare. Wing: vein R4+5 with posterior appendix; vein M1 perpendicular to vein R4+5; postero-apical corner of cell r4+5 widely rounded to rectangular, with or without small appendix; crossvein r-m located between basal 1/6 to 1/3 of cell dm. Abdomen elongate, more than three times as long as wide, constricted, with narrowest point between halfway tergite 2 and transition between tergites 2 and 3. Tergites 3 and 4 fused. Sternite 1 pilose or bare. Male genitalia: phallus furcate near apex, with dorsal process long and whip-like, ventral process very short; epandrium with ventrolateral ridge.
Vein R4+5 with posterior appendix. Vertex convex and shining, sparsely pilose to bare. Abdomen petiolate, except parallel-sided in Pseudomicrodon biluminiferus Hull, but tergite 2 distinctly dorsoventrally flattened in that species.
Among Microdontinae with a petiolate abdomen, Pseudomicrodon species are recognized by their convex and shining vertex. Microdon biluminiferus Hull, 1944 is the only included species without a petiolate abdomen. Instead, the abdomen is parallel-sided, but in lateral view appears constricted because of the dorsoventrally flattened segment 2. This species is assigned to Pseudomicrodon based on the convex vertex and the morphology of the male genitalia (Figs 319, 320), combined with the results of the phylogenetic analysis of morphological characters (Reemer and Ståhls in press).
At present, the basis for distinguishing Ceriomicrodon, Pseudomicrodon and Rhopalosyrphus is narrow. The groups are certainly related, but as presently defined it is doubtful whether they are monophyletic, considering the variation in several morphological characters.
Keiser (1971) described Pseudomicrodon elisabethae from Madagascar. This species is here included in Paramixogaster. Cheng and Thompson (2008) mention the similarity of the South African taxon Microdon illucens Bezzi, 1915 to Pseudomicrodon, which is here also included in Paramixogaster.
Described species: 15. Neotropical.
http://species-id.net/wiki/Ptilobactrum
Figs 321–326Body length: 13 mm. Broadly built flies with very wide head, long antennae and orange markings on abdomen. Head wider than thorax. Face much wider than eye; dorsally with oblique groove from lunule to eye margin; convex in profile. Lateral oral margins weakly produced. Vertex not swollen, more or less flat, but much wider than eye. Occiput narrow ventrally, moderately widened dorsally. Eye bare. Eye margins in male not converging at level of frons, with mutual distance about seven times width of antennal fossa. Antennal fossa somewhat higher than wide. Antenna longer than height of head. Basoflagellomere five times as long as scape; with long pilosity. Postpronotum pilose. Mesoscutum with transverse suture incomplete. Scutellum without calcars. Anepisternum with deep sulcus; entirely pilose. Anepimeron entirely pilose. Katepimeron convex; smooth; bare. Wing: vein R4+5 with posterior appendix; vein M1 straight, somewhat recurrent; postero-apical corner of cell r4+5 angular, with small appendix; crossvein r-m located around basal 1/3 of cell dm. Abdomen oval, widest at posterior margin of tergite 2. Tergites 3 and 4 fused. Sternite 1 bare. Sternite 4 in male visible from below. Male genitalia: phallus bent dorsad, except extreme apex bent ventrad; phallus furcate near apex; epandrium without ventrolateral ridge; surstylus broad, unfurcate, with short posterior lobe. Female unknown.
Vein R4+5 with posterior appendix. Basoflagellomere with long pile. Abdomen oval. Tergites 3 and 4 fused.
Bezzi (1915) distinguished Ptilobactrum from Microdon species by the “breadth of the head, the face being furrowed, and by the unusual shape of the antennae.” Indeed, the grooves on the face, running fom the lunula obliquely downward to the eye margins, are quite unusual among Microdontinae. They are reminescent of the ptilinal sutures of Diptera Schizophora. Similar grooves are found in certain species of Furcantenna, Schizoceratomyia, Paramixogaster and Thompsodon, but usually less distinct. The antennae are unusual in their long pilosity, a character shared with Ceratrichomyia, Furcantenna, Kryptopyga and Schizoceratomyia.
See Ceratrichomyia for a discussion on synonymy of that genus with Ptilobactrum, as proposed by Cheng and Thompson (2008).
Described species: 1. Afrotropical, only known from Kenya.
http://species-id.net/wiki/Rhoga
Figs 327–331Body length: 5–10 mm. Stingless bee mimicking flies with short to moderately long antennae and oval, kite-shaped or more or less parallel-sided abdomen. Head slightly wider than thorax. Face convex; narrower than an eye. Lateral oral margins not produced. Vertex narrow, convexly produced and shining in most species, flat in some. Occiput wide and parallel-sided over entire length. Eye with short, sparse pile. Eye margins in male not converging at level of frons, with mutual distance 2 to 3 times as large as width of antennal fossa. Antennal fossa about as wide as high. Antenna as long as or shorter than distance between antennal fossa and anterior oral margin; basoflagellomere shorter to longer than scape, oval; bare. Postpronotum pilose. Scutellum semicircular, in some species weakly sulcate apicomedially; without calcars. Anepisternum without sulcus; pilose anterodorsally and posteriorly, widely bare in between. Anepimeron entirely pilose. Katepimeron convex; bare. Metapleurae either separated or forming postmetacoxal bridge. Wing: vein R4+5 without posterior appendix; vein M1 perpendicular to vein R4+5; postero-apical corner of cell r4+5 rectangular, with small appendix; crossvein r-m located within 1/4 of cell dm, usually within basal 1/10. Abdomen oval or kite-shaped, 1.5 to 2.5 times as long as wide. Tergites 3 and 4 fused. Sternite 1 pilose or bare. Male genitalia: phallus furcate near apex, with dorsal and ventral process equally long; epandrium without ventrolateral ridge.
Vein R4+5 without posterior appendix. Occiput widened and parallel-sided over entire length.
In some species (e.g. Rhoga mellea (Curran, 1940), Rhoga maculata (Shannon, 1927)) the metapleura are separated and do not form a postmetacoxal bridge. So far in Microdontinae, this character state was known only in the genus Spheginobaccha (Cheng and Thompson 2008).
The type specimen of the type species, Rhoga lutescens Walker, 1857, is not present in the BMNH-collection (pers. comm. N. Wyatt), where it is supposed to be according to Thompson et al. (1976) and Thompson (2010). Apparently it is lost.
Described species: 5. Central and South America. Several undescribed species are known to the first author.
http://species-id.net/wiki/Rhopalosyrphus
Figs 332–355Body length: 9–15 mm. Slender flies with long antennae and petiolate abdomen. Head a little wider than thorax. Face more or less convexly produced on ventral half; narrower than an eye. Lateral oral margins produced. Vertex flat, entirely pilose. Occiput ventrally narrow, dorsally strongly widened. Eye bare. Eye margins in male converging at level of frons, with mutual distance 1-2 times width of antennal fossa. Antennal fossa about 1.5 times as wide as high. Antenna longer than distance between antennal fossa and anterior oral margin; basoflagellomere longer than scape, oval; bare. Postpronotum pilose. Scutellum semicircular; with or without calcars, if present, then small and with mutual distance small. Anepisternum convex or with weak sulcus; entirely pilose. Anepimeron entirely pilose. Katepimeron flat to weakly convex; with wrinkled texture; bare, partly pilose or entirely pilose. Wing: vein R4+5 with posterior appendix; vein M1 perpendicular to vein R4+5; postero-apical corner of cell r4+5 widely rounded to rectangular, with or without small appendix; crossvein r-m located between basal 1/8 to 1/4 of cell dm. Abdomen elongate, more than three times as long as wide, constricted, with narrowest point between halfway tergite 2 and transition between tergites 2 and 3. Tergites 3 and 4 fused. Sternite 1 pilose or bare. Male genitalia: phallus furcate near apex, with dorsal process long and whip-like, ventral process very short; epandrium with ventrolateral ridge.
Vein R4+5 with posterior appendix. Abdomen petiolate. Vertex flat, entirely pilose. Postpronotum pilose. Mesonotal transverse suture incomplete. Tergites 3 and 4 fused. Anterior margin of tergite 2 at least twice as wide as posterior margin. Rhopalosyrphus s.s.: katepimeron pilose. Rhopalosyrphus s.l.: katepimeron bare.
Previous authors have defined this genus more strictly than is done in the present paper. Weems et al. (2003) and Cheng and Thompson (2008) only included species with a pilose katepimeron. A number of additional species are known from the Neotropical region which are similar to Rhopalosyrphus auct. in most characters, but which have a bare or almost bare katepimeron. In Rhopalosyrphus robustus sp. n. the katepimeron is only narrowly pilose along the anterior margin. In all other characters, this species has the diagnostic characters of Rhopalosyrphus as described by Weems et al. (2003): abdomen petiolate, antenna longer than face, scape and basoflagellomere elongate, face produced ventrally (variable), occiput strongly widened dorsally, metasternum developed, hind tibia flared apically. The male genitalia of Rhopalosyrphus robustus are very similar to those of Rhopalosyrphus auct., with an apically furcate phallus, of which the dorsal process is very long and whip-like (Figs 352-355).
Microdon abnormis Curran, 1925 is also similar to Rhopalosyrphus in the characters mentioned above, but has a bare katepimeron. In the analysis of morphological characters by Reemer and Ståhls (in press), a closely related species (Rhopalosyrphus abnormoides sp. n.) is placed within Rhopalosyrphus.
Based on the results of the phylogenetic analyses and the (subjective) evaluation of external and genitalic characters, Rhopalosyrphus is here extended to include also the species with a bare or almost bare katepimeron, which includes species previously grouped in the abnormis group (see account of Pseudomicrodon in Cheng and Thompson 2008), as well as Microdon cerioides Hull, 1943. Species with a pilose katepimeron are included in Rhopalosyrphus s.s., while the other species are treated as Rhopalosyrphus s.l.
The inclusion of Rhopalosyrphus oreokawensis sp. n. in this genus is to be regarded as preliminary. Unlike the other species included in Rhopalosyrphus, this species has very short antennae, an oblique vein M1 and a more slender tergite 2. Analysis of its morphological characters (Reemer and Ståhls in press) resolves its phylogenetic position near Rhopalosyrphus. Possibly, it would be better to erect a new genus for this species. This is nevertheless not done here, in awaitance of a better understanding of the relationships of the taxa included in the ‘Rhopalosyrphus-clade’.
Described species: 9. Mainly Neotropical, with two species in southern parts of the U.S.A. (Arizona, Texas, Florida).
http://species-id.net/wiki/Schizoceratomyia
Figs 356–363Body length: 4–9 mm. Broadly built flies with long antennae (bifrucate in male) and oval abdomen. Head wider than thorax. Face slightly convex or medially concave; wider than an eye. Mouth parts weakly developed, small; oral opening small and round, with lateral margins not produced. Vertex more or less flat, not strongly produced or convex. Frontal ocellus normal, split in two medially or absent. Occiput ventrally narrow, dorsally weakly widened. Eye bare. Eyes in male not or only slightly converging at level of frons, with mutual distance 3–4 times the width of antennal fossa. Antennal fossa about as wide as high. Antenna longer than distance between antennal fossa and anterior oral margin. Basoflagellomere longer than scape, bifurcate in male, in some species also in female; both branches long pilose, especially on inner side; in one (undescribed) species occupied with more than 20 long, narrow tubercles. Arista in male well-developed (longer than pedicel) or reduced to a small stump (shorter than pedicel); in female well-developed, sometimes almost as long as basoflagellomere and thickened. Postpronotum pilose or bare. Scutellum semicircular; without calcars. Anepisternum convex, sometimes with weak sulcus in dorsal 1/4; pilose on dorsal 2/3 to 3/4. Anepimeron pilose on dorsal 3/4 to 1/4, or only along posterior margin. Katepimeron convex; bare; smooth. Wing: vein R4+5 without posterior appendix; vein M1 straight and perpendicular to vein R4+5, or with weak outward angle in anterior 1/2; postero-apical corner of cell r4+5 rectangular to widely rounded, with or without small appendix; crossvein r-m located between basal 1/8 and 1/4 of cell dm. Abdomen dorsoventrally flattened; more oval, with largest width at tergite 3; 1.5-2 times as long as wide. Tergites 3 and 4 fused. Male genitalia: phallus furcate near apex, straight or apically bent ventrad, projecting not or hardly beyond apex of hypandrium; hypandrium with bulb-like base; epandrium without ventrolateral ridge; surstylus unfurcate, elongate or wide.
Vein R4+5 without posterior appendix. Abdomen oval. Antenna longer than distance between antennal fossa and anterior oral margin. Antenna inserted below dorsal eye margin. Vertex more or less flat. Katepisternum bare. Metasternum bare.
Hull (1949) and Papavero (1962) treated Schizoceratomyia as a synonym of Masarygus. See Masarygus for discussion on this synonymy, which is not followed here. These authors, as well as Cheng and Thompson (2008) also considered Johnsoniodon as a synonym of Schizoceratomyia, as is also done in the present paper. Although in the two species originally included in Schizoceratomyia (Schizoceratomyia barretoi Carrera, Lopes & Lane, 1947 and Schizoceratomyia flavipes Carrera, Lopes & Lane, 1947) the basoflagellomere is bifurcate in the male only, whereas in Johnsoniodon this character is found in the female, these taxa are otherwise very similar.
Apparently, Curran (1947) was unaware of the description of Schizoceratomyia by Carrera et al. (1947a, b) when his description of Johnsoniodon malleri Curran, 1947 was published, as this happened almost simultaneously. According to Van Doesburg (1966), the name Schizoceratomyia was published on the 3rd of July 1947, and Johnsoniodon on 14th of July 1947. Cheng and Thompson (2008) stated that Schizoceratomyia was published on the 12th of July 1947. Regardless of whether the date is 3rd or 12th of July Schizoceratomyia has priority over Johnsoniodon.
Besides Schizoceratomyia malleri (Curran), a furcate basoflagellomere in the female is also found in Masarygus carrerai Papavero, 1962. This species is also included in Schizoceratomyia.
Remarkably, in some specimens of Schizoceratomyia, the frontal ocellus is split in two, strongly reduced or even absent, whereas the posterior ocelli are well-developed. Following present species definitions, different states for this character seem to occur within the same species. However, more taxonomic work at species-level is necessary to establish whether this character state variation is intra- or inter-specific. In most Diptera and other flying insects, all three ocelli are well-developed. Reduced or absent ocelli occur in certain terrestrial insects, like certain ants and cockroaches. Among Diptera, they are partly or entirely absent only in a few groups, apparently mainly in certain nematocerous families and some brachypterous or apterous taxa (Cumming and Wood 2009). It will be interesting to try to correlate the degree of development of the frontal ocellus to behaviour and life-history of Schizoceratomyia-species; aspects which are currently unknown, unfortunately.
Described species: 4. Neoptropical. A few undescribed species are known to the first author.
http://species-id.net/wiki/Serichlamys
Figs 364–376Body length: 7–13 mm. Small to medium-sized flies, black, brownish or metallic green, with moderately short to long antennae and oval abdomen. Head about as wide as thorax or slightly wider. Face convex; about as wide as an eye or narrower. Lateral oral margins not produced. Vertex flat. Occiput ventrally narrow, dorsally widened. Eye bare or pilose. Eye margins in male converging at level of frons, with mutual distance two to four times as large as width of antennal fossa. Antennal fossa about as wide as high. Antenna shorter to longer than distance between antennal fossa and anterior oral margin; basoflagellomere shorter to longer than scape, oval or slightly sickle-shaped with swollen base, with rounded apex; bare. Postpronotum pilose. Scutellum semicircular; with narrow, elongated calcars, often quite parallel and with small mutual distance, sometimes dorsoventrally flattened. Anepisternum weakly sulcate; pilose anteriorly and posteriorly, widely bare ventrally and medially. Anepimeron entirely pilose. Katepimeron convex; smooth or with wrinkled texture; bare. Wing: vein R4+5 with posterior appendix; vein M1 perpendicular to vein R4+5; postero-apical corner of cell r4+5 rectangular or weakly rounded, always with small appendix; crossvein r-m located between basal 1/5 to 1/3 of cell dm. Abdomen oval, about 1.5 to 2 times as long as wide. Tergites 3 and 4 fused. Sternite 1 pilose or bare. Male genitalia: phallus furcate, with furcation point near apex; hypandrium with basal part bulb-like; epandrium without ventrolateral ridge.
Vein R4+5 with posterior appendix. Abdomen oval. Vertex flat. Occiput dorsally (slightly) widened. Postpronotum pilose. Scutellum with calcars. Postero-apical corner of cell r4+5 rectangular, with small appendix. Proepimeron pilose. Anepisternum widely bare medially, also on dorsal half. Anepimeron entirely pilose. Male genitalia: phallus furcate near apex.
Curran (1925a) erected Serichlamys as a subgenus of Microdon, but subsequent authors considered Serichlamys as a synonym of the typic subgenus of Microdon (Wirth et al. 1965, Thompson 1981b, Cheng and Thompson 2008). Curran (1925a) did not clearly state which characters he considered diagnostic. In his key, Curran keyed out the type species Microdon rufipes (Macquart, 1842) by its eyes being pilose, which was based on a translation of the original description of Aphritis rufipes. Indeed, Macquart (1842) wrote that this species has ‘yeux peu velus’ (eyes little pilose). However, examination of the type specimen (coll. OUMNH) revealed that its eyes are bare. Either pile have been wiped off or eroded in the course of time, or Macquart (1842) made an error in his description. Whether Aphritis rufipes has pilose eyes or not, Serichlamys is here recognized as distinct as all included species differ in other characters from Microdon s.s., e.g. postero-apical corner of cell r4+5 rectangular (rounded in Microdon s.s.), phallus furcate apically, hypandrium with bulb-like base.
The differences with Microdon s.s. could be used as arguments for reinstating the subgeneric status of Serichlamys. However, a subgeneric status is contradicted by the phylogenetic results of Reemer and Ståhls (in press), who recovered two Neotropical species of Serichlamys as sister group to Archimicrodon, without apparent close affinities to Microdon. The type species Serichlamys rufipes and Serichlamys scutifer (Knab, 1917) were included in a phylogenetic analysis based only on morphology, and placed in a large and rather uninformative polytomy, but not within a clade containing species of Microdon s.s. For this reason, Serichlamys is here raised to genus level.
Three Nearctic species are included in Serichlamys: Serichlamys rufipes (Macquart, 1842), Serichlamys scutifer (Knab, 1917), and Serichlamys diversipilosus (Curran, 1925). The latter species is included with uncertainty, based only on the description, as no specimens were examined. Two Neotropical species are included: Serichlamys mitis (Curran, 1940) and Serichlamys mus (Curran, 1936). The Neotropical species differ from the Nearctic ones in the shape of the surstylus, which has a long posterior process which is lacking in the Nearctic species. Otherwise the species are very similar. Species of Serichlamys quite similar to the Old World genus Archimicrodon in general habitus and important morphological characters, including the male genitalia. Generally, the antennae of Serichlamys-species are longer and the scutellar calcars are longer.
Described species: 4 or 5. Nearctic (2 or 3 described species) and Neotropical (2 described species). Several undescribed species from the Neotropical regioan are known to the first author.
http://species-id.net/wiki/Spheginobaccha
Figs 377–387Body length: 7–19 mm. Slender flies with short antennae and constricted abdomen. Head about as wide as to wider than thorax. Face in profile straight to slightly concave in dorsal 2/3, with a faint convex tubercle in ventral 1/3; narrower than an eye. Lateral oral margins not produced. Vertex flat. Occiput narrow ventrally, widening dorsally, with distinct crease in dorsal 2/3. Eye bare (African species) or short pilose (Oriental species). Eyes in male not (African species) or strongly (Oriental species) converging at level of frons, in one Oriental species (Spheginobaccha chilcotti Thompson) even nearly contiguous. Antennal fossa about twice as wide as high. Antenna shorter than distance between antennal fossa and anterior oral margin. Basoflagellomere longer than scape, oval, except more or less triangularly enlarged in males of some African species; bare. Postpronotum pilose. Scutellum semicircular; without calcars. Anepisternum without sulcus; entirely sparsely pilose, sparsely pilose only posteriorly, or entirely bare. Anepimeron pilose on dorsal half or bare. Katepimeron flat; bare or pilose; smooth. Wing vein R4+5 without posterior appendix. Vein M1 oblique and more or less parallel to wing margin, in African species only so in anterior 1/2, posterior 1/2 straight. Postero-apical corner of cell widely rounded and without appendix in Oriental species, rectangular and with appendix in African species. Crossvein r-m located between basal 1/6 to 1/3 of cell dm. Abdomen constricted, narrowest halfway or at posterior margin of tergite 2, widest at tergite 4. Tergites 3 and 4 fused. Male genitalia: phallus unfurcate, straight (African species) or bent dorsad (Oriental species), articulating with hypandrium apically (perialla-group) or basally (macropoda- and rotundiceps-group); hypandrium with apical part consisting of separate lobes; epandrium without ventrolateral ridge; surstylus unfurcate, oval or more or less rectangular to triangular.
Metapleura not connected, not forming a postmetacoxal bridge. Abdomen constricted. Occiput with deep crease on dorsal 2/3.
Hull (1949) was the first to include Spheginobaccha in the Microdontinae. Thompson (1969) excluded it, after which Ståhls et al. (2003) included it again. The latter placement was based on a sister-group relationship of Spheginobaccha to all other Microdontinae, as recovered in a phylogenetic analysis of combined molecular and morphological characters. Species can be identified using Thompson (1974), supplemented with Dirickx (1995).
Dexiosyrphus was described by Hull (1949) as a subgenus of Spheginobaccha, based on Spheginobaccha rotundiceps (Loew, 1857). Thompson (1974) argued that if Dexiosyrphus was to be recognized, then another subgenus would have to be erected for the perialla-group. He considered this unnecessary, as the three species groups he recognized were sufficient for proper segregation of the species. We see no reason to adopt a different point of view.
Described species: 16. Oriental (10 species) and Afrotropical (6 species). Oriental records range from Nepal through Burma, Thailand and Vietnam to Java and Borneo. Afrotropical records are from Malawi, South Africa and Madagascar.
http://species-id.net/wiki/Stipomorpha
Figs 388–396Body length: 6–11 mm. Stingless bee mimicking flies with moderately long antennae and more or less triangular abdomen. Head slightly wider than thorax. Face in profile straight to convex; narrower to wider than an eye. Lateral oral margins hardly to moderately produced. Vertex flat, convex or irregularly swollen. Occiput narrow ventrally, slightly widened dorsally. Eye bare. Eye margins in male converging at level of frons, with mutual distance 1–3 times width of antennal fossa. Antennal fossa about as wide as high. Antenna shorter to longer than distance between antennal fossa and anterior oral margin; basoflagellomere shorter to longer than scape, oval; bare. Postpronotum pilose. Scutellum semicircular, sometimes weakly sulcate apicomedially; without calcars. Anepisternum convex, without sulcus; anterodorsally pilose, posteriorly pilose or bare, widely bare in between. Anepimeron with pile limited to dorsal half, if pilose on ventral half then only sparsely. Katepimeron convex; bare. Wing: vein R4+5 usually with posterior appendix (seldomly missing); vein M1 perpendicular to vein R4+5; postero-apical corner of cell r4+5 widely rounded to rectangular, with or without small appendix; crossvein r-m located between basal 1/5 to 1/3 of cell dm. Abdomen widest at tergite 2, with next tergites either gradually narrowing (kite-shaped abdomen) or more or less parallel-sided; 1.5 to 3.5 times as long as wide. Antetergite almost fused to tergite 1; in most species enlarged, concave and smooth. Tergites 3 and 4 fused. Sternite 1 bare. Male genitalia: phallus unfurcate, bent dorsad, in most species projecting beyond apex of hypandrium; hypandrium with bulb-like base; epandrium without ventrolateral ridge; surstylus in most species with two wide lobes, but other shapes also occur.
Sternites 2 and 3 separated by membraneous part as wide as or wider than sternite 2.
When Hull (1945) erected Stipomorpha as a subgenus of Microdon, he did so based on the shape of the abdomen: “...the first two abdominal segments greatly flared and flattened and wider than the thorax; remainder of the abdomen immediately compressed into a rounded, subcylindrical pipe-like form.” Shortly after, Hull (1949) ranked Stipomorpha as a subgenus of Paramixogasteroides Shiraki, 1930, without stating a reason for this. Subsequent authors have regarded Stipomorpha as synonymous with Ubristes. See under Ubristes for a discussion on the relationship between these groups, which are here considered as separate genera. Stipomorpha as presently defined contains most species listed under Ubristes by Thompson et al. (1976).
Described species: 16. Descriptions of nine additional species are in preparation by the first author. Neotropical, with records ranging from Costa Rica to Argentina.
urn:lsid:zoobank.org:act:1E585145-3AD1-4FCE-8A29-4B18F170E98F
http://species-id.net/wiki/Sulcodon
Figs 397–399Body length: 7–9 mm. Broadly built flies with moderately long antennae and short abdomen. Head about as wide as thorax or slightly wider. Face convex; about as wide as an eye. Lateral oral margins distinctly produced. Vertex irregularly swollen. Occiput ventrally narrow, dorsally widened. Eye bare. Eye margins in male converging at level of frons, with mutual distance 2.5 times as large as width of antennal fossa. Antennal fossa about as wide as high. Antenna longer than distance between antennal fossa and anterior oral margin; basoflagellomere about as long as to slightly longer than scape, parallel-sided; bare. Postpronotum bare. Scutellum semicircular; with large, blunt calcars, separated by deep sulcus. Anepisternum weakly sulcate; entirely pilose. Anepimeron entirely pilose. Katepimeron flat; bare. Wing: vein R4+5 with posterior appendix; vein M1 perpendicular to vein R4+5; postero-apical corner of cell r4+5 rectangular, with small appendix; crossvein r-m located around basal 1/4 of cell dm. Abdomen heart-shaped, about as long as wide. Tergites 3 and 4 fused. Sternite 1 bare. Male genitalia: phallus furcate, with furcation point near apex; hypandrium with basal part bulb-like; epandrium without ventrolateral ridge; surstylus deeply furcate.
Postpronotum bare. Abdomen about as long as wide, with tergite 2 about as long as tergites 3 and 4 together.
The only species included in this group, the Oriental Microdon sulcatus Hull, 1944, does not have any obvious relatives. Because of the bare postpronotum, the rectangular postero-apical corner of cell r4+5, the entirely pilose anepisternum and the characters of the male genitalia, the species does not fit into Microdon s.s.
Described species: 1. Indonesia: Java. The species seems not to be uncommon, as specimens collected by different collectors in different years are present in several entomological collections (BMNH, KBIN, MZH, RMNH, ZMAN). Although entomological collectors have been active in other parts of the Sunda region, such as peninsular Malaysia, Sumatra and Borneo, this species has so far not been found there. This suggests that this singular species is endemic to Java.
Body length: 4–5 mm. Small flies with short antennae and oval abdomen. Head slightly wider than thorax. Face convex; narrower than an eye. Lateral oral margins not produced. Vertex flat. Occiput ventrally narrow, dorsally widened. Eye bare. Eye margins in male not converging at level of frons, with mutual distance about 3 times as large as width of antennal fossa. Antennal fossa about as wide as high. Antenna shorter than distance between antennal fossa and anterior oral margin; basoflagellomere shorter to longer than scape, oval, about twice as long as wide, bare. Postpronotum bare. Scutellum semicircular; without calcars. Anepisternum convex; dorsally with thick, setae-like pile, ventrally bare. Anepimeron dorsally with thick, setae-like pile, ventrally bare. Katepimeron convex; bare. Wing: vein R4+5 without posterior appendix; vein M1 straight, perpendicular to vein R4+5; postero-apical corner of cell r4+5 rectangular, with or without small appendix; crossvein r-m located very close to base of cell dm. Abdomen oval, about 1.5 times as long as wide. Tergites 3 and 4 fused. Sternite 1 bare. Male genitalia: phallus furcate, with furcation point about halfway, curved dorsad, straight, projecting not or slightly beyond apex of hypandrium; hypandrium without bulb-like base; epandrium without ventrolateral ridge; surstylus unfurcate.
Abdomen oval; yellow and black. Vein R4+5 without posterior appendix. Postpronotum bare. Antenna shorter than distance between antennal fossa and anterior oral margin.
Reemer (2008) included in his new genus Surimyia a species previously assigned to Paragodon (Paragodon minutula van Doesburg, 1966). Several morphological characters were mentioned to indicate the differences between these genera (Reemer 2008). Especially the structure of the phallus seems fundamentally different: short, straight and unfurcate in Paragodon, and long, curved and bifurcate in Surimyia. Other distinctive differences are the bare postpronotum in Surimyia (pilose in Paragodon) and the bare anatergum in Surimyia (microtrichose in Paragodon).
Described species: 2. Neotropical (presently only known from Surinam).
urn:lsid:zoobank.org:act:C5C25319-6F81-42B6-A805-1B897435AC8B
http://species-id.net/wiki/Thompsodon
Figs 406–416Thompsodon conspicillifrons Reemer spec. n. Type locality: Costa Rica.
Body length: 8 mm. Moderately slender flies with long antennae and basally constricted abdomen. Face in profile slightly convex, almost straight; laterally weakly depressed, therefore slightly carinate dorsomedially; about as wide as an eye. Lateral oral margins not produced. Frons laterally with round, concave areas, filled with dense golden pile, ventrally delimited by a sharply defined ridge. Vertex irregularly swollen. Occiput narrow ventrally, strongly widened dorsally. Eye bare. Antennal fossa about as high as wide. Antenna longer than distance between antennal fossa and anterior oral margin; basoflagellomere about as long as scape; elongate, with dorsal margin straight and ventral margin convex, apex slightly acute. Postpronotum pilose. Anepisternum with shallow sulcus; entirely long pilose. Anepimeron entirely pilose. Katepimeron weakly convex; bare; with wrinkled texture. Scutellum semicircular, weakly triangular; without calcars. Wing: vein R4+5 with posterior appendix; vein M1 perpendicular to vein R4+5; postero-apical corner of cell r4+5 rectangular, with small appendix; crossvein r-m located around basal 1/7 of cell dm. Abdomen constricted at tergite 1, narrowest at tergite 1, widest at posterior margin of tergite 3. Tergites 3 and 4 not fused, able to articulate independently.
Frons laterally with round, concave areas, filled with dense golden pile, ventrally delimited by a sharply defined ridge. Transverse suture complete. Tergites 3 and 4 not fused.
The only known specimen representing this genus has some characters that are not often found among Microdontinae: mesonotal transverse suture complete, tergites 3 and 4 not fused. The lateral concave and densely golden pilose areas on the frons, which are ventrally delimited by a sharply defined ridge, are even unique within the subfamily. The unfused tergites 3 and 4 may suggest affinity with the Neotropical Ceratophya or the Oriental Kryptopyga, whereas the complete transverse suture reminds of Ceratrichomyia andIndascia. However, the specimen does not agree with the diagnoses of any of these genera, so a new genus seems the best way to make sure that this singular taxon will get the attention it deserves in future studies on the taxonomy of Microdontinae. Hopefully, male specimens will be collected in the near future, which can be used for study of the male genitalia and molecular analyses.
Described species: 1. Only known from Costa Rica.
This genus is dedicated to Dr. F. Christian Thompson, in acknowledgement of the valuable work he has done on the taxonomy of the Syrphidae in general, and the Microdontinae in particular.
http://species-id.net/wiki/Ubristes
Figs 417–421Body length: 10–11 mm. Slender flies with long antennae and long, brush-like pilosity on hind tibiae. Mimics of Trigona-like stingless bees. Head wider than thorax. Face slightly convex, almost straight in lateral view; wider than eye. Lateral oral margins produced. Vertex flat. Occiput ventrally narrow, dorsally widened. Eye very sparsely and short pilose, appearing bare under low magnification. Eye margins in male converging at level of frons; mutual distance about three times width of antennal fossa. Antennal fossa about as high as wide. Antenna longer than distance between antennal fossa and anterior oral margin; basoflagellomere longer than scape. Postpronotum pilose. Anepisternum sulcate; pilose anteriorly and posteriorly, widely bare in between. Katepimeron convex; bare. Scutellum semicircular; without calcars. Wing: vein R4+5 with posterior appendix; vein M1 perpendicular to vein R4+5; cell r4+5 with postero-apical angle widely rounded; crossvein r-m located between basal 2/5 and 1/2 of cell dm. Hind tibia with long, brush-like pilosity. Abdomen elongate: parallel-sided or somewhat triangular. Tergite 2 with lateral tubercle at half of length. Tergites 3 and 4 fused. Sternites 1, 2 and 3 not separated by very wide membranes. Male genitalia: phallus furcate basally; epandrium with lateral ‘fenestrae’: well-defined, translucent, oval depressions; surstylus more or less oval.
Hind tibia with long, brush-like pilosity. Scutellum without calcars. Vein R4+5 with appendix. Tergite 2 with lateral tubercle at half of length.
Thusfar, Ubristes has been characterized by the brush-like pilosity of the hind tibia, giving the flies the appearance of stingless Trigona-like bees (Cheng and Thompson 2008, Thompson et al. 1976). Based on this definition, 31 species were assigned to this group by Thompson et al. (1976), including the type species of Carreramyia, Hypselosyrphus and Stipomorpha. The latter two groups were considered as ‘subgroups’ of Ubristes by Cheng and Thompson (2008), because the characters previously used to define the groups (abdominal shape) were considered of little taxonomic value.
Closer examination of the morphology reveals several important differences between these taxa. The structure of the male genitalia of Ubristes is very different from those of the species here included in Carreramyia, Hypselosyrphus and Stipomorpha: the phallus is long and slender and furcate near its base, the base of the hypandrium is not bulged and there are well-defined, translucent, oval lateral depressions in the epandrium (here called ‘fenestrae’). In external morphology Ubristes is readily distinguished from the mentioned genera by e.g. the lateral tubercles on tergite 2. For other differences see the accounts of the other taxa. Considering the phylogenetic results of Reemer and Ståhls (in press) and the morphological differences between these taxa, Ubristes sensu Thompson et al. (1976) and Cheng and Thompson (2008) is here considered to be polyphyletic, with Carreramyia, Hypselosyrphus and Stipomorpha each as separate lineages. Besides the type species, two undescribed species are assigned to Ubristes.
Thompson et al. (1976) and Cheng and Thompson (2008) ranked Ubristes as a subgenus of Microdon. However, the species of Ubristes differ in several characters from the species of Microdon s.s., as defined in the present paper. Here, the view is taken that it is better to treat Ubristes as a genus instead of a subgenus, in order to make sure that Microdon comprises less heterogeneous groups with uncertain affinities.
Described species: 1. Descriptions of two additional species are in preparation by the first author. Central and South America.
Basoflagellomere strongly widened, more or less triangular. Arista pilose.
This species has first been recognized as undescribed by Dr. F.C. Thompson, who is preparing a description of it. As the species posesses some unique characters not found in any other species of Microdontinae, the present authors feel that it belongs in a new genus. These characters are: basoflagellomere strongly widened and more or less triangular; arista pilose; phallus dorsobasally with long projection; epandrium with dorsolateral ridge. Other interesting characters are the undeveloped mouthparts (shared with Masarygus) and the lateral carinae on the face.
Described species: 1. Australia (Queensland).
Basoflagellomere bifurcate. Abdomen more or less parallel-sided, slightly constricted between tergites 2 and 3.
This species has first been recognized as undescribed by Dr. F.C. Thompson, who is preparing a description of it. This taxon resembles Carreramyia in the bifurcate antenna, the wing venation and the structure of the male genitalia. It differs from that genus by the more or less flat vertex (strongly produced in Carreramyia), the short pilose hind tibia (long pilose in Carreramyia), and the more or less parallel-sided, slightly constricted abdomen (triangular in Carreramyia).
Only known from one species, collected in Costa Rica.
A small number of species is left unclassified. These are listed at the end of the following section on species classification. On a few of these taxa, comments are given below.
Based on external characters, no close relatives were recovered in the phylogenetic analysis of morphological characters by Reemer and Ståhls (in press). The species is characterized by its metallic blue colouration and golden pilosity, a long basoflagellomere, a medially widely bare face, a rectangular postero-apical corner of wing cell r4+5, and unfused tergites 3 and 4. The latter character may indicate affinity with Ceratophya, Kryptopyga or Thompsodon, but the species lacks other diagnostic characters for these taxa. This species is left unplaced for now.
Whether this taxon belongs to Microdontinae or Syrphidae at all is uncertain. It was described from larvae found in an ants nest (Wheeler 1924). Cheng and Thompson (2008) suspect it belongs to another family, perhaps Phoridae. Photographs of a larva can be found at http://syrphidae.lifedesks.org/pages/25523.
In order to find diagnostic characters for distinguishing Microdontinae from other Syrphidae, characters described by Hippa and Ståhls (2005), Hull (1949), Shatalkin (1975a, b), Speight (1987) and Thompson (1969, 1972) were evaluated based on the material examined for this study. The discussion of these characters below is subdivided into paragraphs corresponding with the following main body parts: head, thorax, wings, legs, abdomen, male genitalia. Terminology of the aforementioned authors is translated into the terminology used in the present paper (see section Material and Methods). This discussion concludes with a summarizing statement on diagnostic morphological characters of Microdontinae.
The simple, convex face of most Microdontinae has been used as a character for the group by Hull (1949) and Thompson (1969, 1972). A facial tubercle is only found in Eurypterosyrphus. In a few taxa (Ceratrichomyia, Chrysidimyia, Rhopalosyrphus) the ventral part of the face is somewhat bulged, but cannot be considered tuberculate. The diagnostic value of this character is limited, as the facial tubercle is also missing in several other Syrphidae, e.g. all Pipizini and Eumerini.
According to Thompson (1969, 1972), the face of Microdontinae is uniformly pilose. In the present study, however, several taxa were found in which the face is bare medially to varying extent (e.g. species of Rhoga, Schizoceratomyia, Stipomorpha), sometimes even entirely bare (e.g. Masarygus planifrons).
Thompson (1972) noted that the anterior oral margin in Microdontinae is not notched. There seems to be some confusion about the interpretation of this character, as Hippa and Ståhls (2005) have coded this character as ‘medially notched’ for Microdon analis (Macquart, 1842). Apparently, the latter authors have regarded the anterior oral margin as notched when the lateral oral margins are produced. Indeed, these laterally produced oral margins give the impression in anterior view that the anterior oral margin is narrowed (notched). In most other Syrphidae (except Pipizini), however, there is a slight additional narrowing at the extreme anterior part of the oral margin (see e.g. fig. 3 in Speight 1987 and fig. 1A in Hippa and Ståhls 2005). This additional narrowing seems to be correlated with the presence of an anteclypeus. In Microdontinae, no distinction can be made between an anteclypeus and a postclypeus (see below), so this additional narrowing of the anterior margin is not visible. In the present study, we follow Hippa and Ståhls (2005) in the interpretation of this character. Many Microdontinae were found with produced lateral oral margins, as it is in most other Syrphidae, so this character is not considered to be useful for distinguishing the subfamily from other Syrphidae.
According to Speight (1987), Microdontinae possess only one clypeus, whereas an anteclypeus and a postclypeus can be recognized in other Syrphidae. The presence of only one clypeus in Microdontinae can be confirmed based on the present study, but the character has not been studied in other Syrphidae. Speight (1987) mentioned two other characters of the mouthparts he considered to be unique for Microdon: 1. the maxillary sclerites are short, flange-like, oriented transversely rather than longitudinally; 2. the maxillary palps are rudimentary. These characters have not been studied in the present study, and thus cannot be commented upon. In general, the mouthparts of Microdontinae are reduced compared with other Syrphidae. No characters indicating the degree of reduction were included in the present study, but a considerable degree of variation was noticed. In certain taxa, the labella are well-developed and flattened, suggesting a capability of feeding on flat surfaces (e.g. leaves) (this can best be noticed in fresh or alcohol-preserved specimens, as the mouthparts tend to shrivel up when dry). In other taxa, the mouthparts are reduced to such an extent that there is not even an oral opening, indicating these species do not feed at all (Masarygus palmipalpus sp. n., Masarygus planifrons).
Unlike most other Syrphidae, the males are dichoptic (i.e. the eyes do not meet at the top of the head). In the present study, no holoptic Microdontinae were found except for Spheginobaccha chillcotti Thompson, 1974, although in a few taxa the male eyes approach each other quite closely (e.g. Hypselosyrphus). When taken into consideration that dichoptic males also occur in other subfamilies of Syrphidae (e.g. Helophilus Meigen, 1822 and related genera, Neoascia Williston, 1887, Pelecocera Meigen, 1822), this character has limited diagnostic value.
According to Thompson (1969, 1972) the arista of Microdontinae is bare. The only known exception, as found in the present study, is the Australian Undescribed genus #1. As a bare arista also occurs in many other Syrphidae, this character is of limited diagnostic value.
A pilose postpronotum has been considered to be an important and stable character for distinguishing Microdontinae (as well as Eristalinae) from Syrphinae (Thompson 1969, 1972). In the present study, the postpronotum was found to be pilose in the majority of Microdontinae, but certainly not in all. The postpronotum is bare in several taxa (e.g. Ceriomicrodon petiolatus, Masarygus, Sulcodon, Surimyia, Paramixogaster, Piruwa, Schizoceratomyia). This needs to be taken into account when using keys to genera of Syrphidae in which this character is used (e.g. Thompson 1999).
A few other characters involving the presence or absence of pile on thoracic sclerites have been used. Thompson (1969, 1972) noted that the anterior part of the anepisternum is pilose in Microdontinae, except in Ceriomicrodon petiolatus. In addition, a bare anterior anepisternum was found in an Aristosyrphus sp. n., a Mixogaster sp. n. and in some species of Spheginobaccha. According to Hull (1949) the metasternum is always pilose in Microdontinae. However, this was only true for slightly more than half of the presently studied taxa. The subscutellar hair fringe was absent in all studied Microdontinae (character of Thompson 1969, 1972). This character also applies to several other Syrphidae (Hippa and Ståhls 2005), so it is not by itself group-defining, although it could be useful in keys.
Another thoracic character considered of importance for Microdontinae (Thompson 1969, 1972) is the presence of a complete ‘postmetacoxal bridge’, formed by the connection of the metapleura. As already observed by Cheng and Thompson (2008), this bridge is lacking in Spheginobaccha. The present study revealed that the metapleura are also distinctly separated in certain species of Rhoga (Rhoga maculata, Rhoga mellea, Rhoga sepulchrasilva). In two other taxa (Paramixogaster variegata) and Surimyia) the metapleura seem to be touching only in one point, implying an intermediate state for this character. Among other Syrphidae, a complete postmetacoxal bridge is rare, but it is found in e.g. Baccha elongata (Fabricius, 1775), Neoascia Williston, 1886 and Sphegina Meigen, 1822 (Hippa and Ståhls 2005), and also in Leucopodella Hull, 1949.
The well-developed plumule, a plumose posterior extension of the subalar sclerite, is considered to be an important character of Syrphidae. In most Syrphinae and Eristalinae the plumule is usually strongly developed, except in Ceriana Rafinesque, 1815, Sphiximorpha Rondani, 1850, Neoascia, Sphegina, Eosphaerophoria Frey, 1946, Allograpa ventralis (Miller, 1921) and some species of Ocyptamus (Hippa and Ståhls 2005, Speight 1987, X. Mengual pers. comm.). As noticed by Thompson (1969, 1972), Speight (1987) and Hippa and Ståhls (2005), the plumule is strongly reduced in Microdontinae. This is confirmed by the results of the present study, although considerable variation was found. In a few taxa, the plumula is entirely absent (e.g. Carreramyia, Masarygus, Spheginobaccha), while in others a short plumula can be found, with both the length of this sclerite and the microtrichosity varying in length.
Speight (1987) drew attention to another character: “At the outer ends of the transverse sulcus of the mesoscutum, Microdon possesses a pair of shelf-like, semi-circular, sclerotized outgrowths of the mesoscutum, which do not seem to have an equivalent in other Syrphids”. This apparently indicates the notal wing lamina, which, however, is also well-developed in certain other syrphids besides Microdon, as noted by Hippa and Ståhls (2005). The present data indicate that the notal wing lamina is undeveloped in several Microdontinae, such as Aristosyrphus, Eurypterosyrphus, Masarygus, Paragodon, Rhoga and species of Hypselosyrphus, Indascia and Paramixogaster. A strongly developed notal wing lamina (in the sense of Hippa and Ståhls 2005) was only found in Chrysidimyia. This character has little diagnostic value for the Microdontinae as a subfamily.
As Speight (1987) noticed, the subscutellum (metanotum) is “unusually flat” in Microdon, whereas in many other Syrphidae often a convex plate is present. This character was found to be variable among Microdontinae, but in this group the subscutellum is never as strongly swollen as in several other Syrphidae. However, as many intermediate states occur, this character cannot be used conveniently as diagnostic at the subfamily level.
The presence of the stigmal crossvein was mentioned as a character of the Microdontinae by Hull (1949) and Thompson (1969). The only exceptions found in the present dataset are Spheginobaccha and Paramicrodon delicatulus Hull, 1937 (the crossvein is present in other studied species of Paramicrodon). A quick but far from exhaustive scan of this character among other Syrphidae learned that the stigmal crossvein is also present in many Eristalinae.
Hull (1949) and Thompson (1969) noted that the apical crossveins M1 and dm-cu are positioned perpendicular to, respectively, vein R4+5 and vein M in most Microdontinae. Exceptions are Aristosyrphus, Mixogaster, Spheginobaccha, and to a lesser extent Kryptopyga and Schizoceratomyia, in which the anterior 1/3 or 1/2 is directed outward. Among other Syrphidae, perpendicular marginal crossveins can be found in e.g. Neoascia and Ocyptamus Macquart, 1834 (subgenus Calostigma Shannon, 1927).
In all Microdontinae, as noticed by Thompson (1969), crossvein r-m is positioned basal of the middle of cell dm. This is not an exclusive character of the subfamily, however, as it is shared with all Syrphinae and many Eristalinae.
An apparently universal character for Microdontinae is the basally curved vein R2+3. The first to introduce this character were Hippa and Ståhls (2005), who noted that the only other Syrphidae in which this character is found are the Cerioidini. No exceptions were found in the present dataset. In the present paper, an attempt is made to describe this important character in a way that makes it easier to judge it objectively (Fig. 435).
The legs of most Microdontinae are marked with clear scars subbasally at the femora and subapically at the tibia, visible as creases surrounding the legs. These scars are named cicatrices, singular cicatrix (Hull 1949, Thompson 1969). In Microdontinae, this character is usually very pronounced, but a few exceptions were found among the studied taxa (e.g. Masarygus palmipalpus, Piruwa phaecada, Schizoceratomyia flavipes). These taxa are small in body size, and cicatrices are present in taxa which are considered closely related (e.g. Schizoceratomyia barretoi). This suggests that the apparent absence of cicatrices might merely be a matter of reduction or reduced visibility of the character. Vague cicatrices can also be seen in several Syrphinae and Eristalinae, although never as clear as in Microdontinae. With these considerations in mind, the character holds a good ‘indicating value’ for diagnosing the subfamily, but it should be applied with caution.
Speight (1987) found that all Syrphidae except Microdon posess a long, blade-like process projecting outwards from the antero-lateral end of the outer side of the posterior mesocoxite, which he termed “trochanteral process of the mesocoxite”. This character has not been examined in the present study.
In Microdontinae, four preabdominal segments are found in the male, as has been noted by many previous authors (e.g. Thompson 1969, Hippa and Ståhls 2005). This character is shared with the Eristalinae, but constitutes a difference with the Syrphinae. No exceptions were found.
Another abdominal character, noted by Thompson (1969) is the position of the first abdominal spiracle, which is embedded in the metaepimeron in Microdontinae. In the present study, this character was confirmed for most taxa. In a few small taxa the character could not be verified because the spiracle could not be found, neither in the metaepimeron nor in the adjacent membranes. The diagnostic value of this character is limited, as the first abdominal spiracle is also embedded in the metaepimeron in many Syrphinae and Eristalinae (Hippa and Ståhls 2005).
The last published characterization of genitalia of Microdontinae is the one of Thompson (1969, with some additional notes in 1972). Although since then the understanding of the homologies of Diptera genitalic structures and their terminology has advanced (McAlpine 1981, Sinclair 2000), the characters listed by Thompson (1969) to distinguish Microdontinae from other Syrphidae are still useful. Some of these characters have also been noticed by other authors (Shatalkin 1975a, b, Speight 1987).
Most of the singularities of the genitalia of Microdontinae are found in the hypandrium (9th sternum) and its associated structures. The hypandrium itself is a simple structure in Microdontinae, lacking separate lobes.
In most taxa, the hypandrium seems to consist of a basal part and an apical part. In certain species this distinction is very clear, because the basal part is convex in lateral view (e.g. Figs 24, 363, 396), but in other ones these parts are smoothly fused and one needs to look carefully to distinguish them (e.g. Figs 171, 179, 319). However, distinction is possible in most cases because the apical part is usually less sclerotized than the basal part and it is covered with very fine microtrichia, while on the basal part these are lacking. There is no doubt that the basal part is the actual hypandrium, because it articulates with the epandrium basolaterally. Possibly, the apical part is homologous to the gonopods of other Diptera, which are usually simple in Muscomorpha and more or less absent in Syrphoidea (McAlpine 1981). In most Microdontinae the apical part consists of one single structure. If this structure is homologous to the gonopods indeed, then this would imply that the gonopods have become fused. In a few taxa (with a basal position in the phylogeny presented in Reemer and Ståhls in press), the apical part of the hypandrium consists of two separate lobes, e.g. in Aristosyrphus (incl. Eurypterosyrphus), Mixogaster and Spheginobaccha (Figs 29, 32, 246, 387). In these cases it is easier to imagine that these structures are homologous to gonopods. In only one studied taxon, Menidon falcatus, no apical part of the hypandrium seems to be present.
No postgonites (parameres of McAlpine 1981, superior lobes of Metcalf 1921 and Hippa and Ståhls 2005) can be distinguished in Microdontinae, a rare occasion among Diptera according to McAlpine (1981). Hippa and Ståhls (2005) supposed that in this subfamily the parameres are integrated into the phallus, but did not present evidence for this hypothesis.
The phallus (subdivided by Thompson 1969 into ejaculatory duct and ejaculatory hood) is tubular and elongate. Its structure is simple: no separate structures can be recognized, as is possible in other Syrphidae (basiphallus, distiphallus etc.). In most taxa, the basal part (termed ‘chitinous box’ in Metcalf 1921 and Thompson 1969) is swollen and spherical (e.g. Figs 29, 363, 385, 405), but in a few this is not obviously so (Figs 22, 386). This basal part might be formed out of the phallapodeme, as Thompson (1974) appears to suggest for Spheginobaccha. However, this seems unlikely, because in other Diptera the phallapodeme does not seem to have a sperm-guiding or -collecting function, while in Microdontinae the spherical base of the phallus clearly has an intermediate position between the sperm duct and the apical part of the phallus. Usually, no external lobes are present, but in some taxa a dorsobasal projection was found (Fig. 22). The aedaegus can be unfurcate or bifurcate. Furcate aedeagi can be divided into a number of types, depending on whether the furcation point is basal or apical, and on the length of the ejaculatory processes (see character nos. 163–165 in Reemer and Ståhls in press).
The phallus, or actually the ejaculatory hood, articulates ventrally with the hypandrium and dorsally with the subepandrial sclerites. The point of articulation with the hypandrium is basal, in contrast with all other Syrphidae. The only studied microdontine taxon in which the phallus was observed to articulate apically with the hypandrium is the African taxon Spheginobaccha guttula Dirickx, 1995, a representative of the perialla-group of Thompson (1974).
Except for the studied African species of Spheginobaccha, Spheginobaccha guttula and Spheginobaccha dexioides Hull, 1944, none of the studied Microdontinae has a clearly recognizable phallapodeme. In the Oriental species of Spheginobaccha this structure is also more or less spherical. According to Thompson (1972), the phallapodeme can be absent or “double” in this subfamily. No explanation is given, but judging from a figure of the genitalia of Microdon manitobensis Curran, 1924 in Thompson and Rotheray (1998) and Vockeroth and Thompson (1987), the phallapodeme in the sense of Thompson corresponds with the dark lines named ‘lateral strips’ in Reemer and Ståhls (in press, character no. 171). This seems unlikely, because the phallapodeme is a single structure while the lateral strips are paired.
Thompson (1969, 1972) pointed out that the ejaculatory apodeme of Microdontinae is ‘triangularly flared’ apically, except in Paragodon, in which it is not sclerotized. The present study has revealed no other taxa with an unsclerotized ejaculatory apodeme. The shape of this structure was found to be very variable, ranging from elongate, round, trapezoid, triangular, square to rectangular. The ejaculatory sac was found to be sclerotized in all taxa except Paragodon and Surimyia. This structure is also variable in shape.
No characters useful for diagnostic purposes at subfamily level were found in the epandrium and associated structures. The shapes of the cerci and surstyli are highly variable, so much even that it is difficult to use them at generic level.
When the characters of Microdontinae described by previous authors are studied across a large set of taxa, as has been done in the present study, exceptions can be found for almost all of them. Characters for which no or few exceptions were found are listed in Table 2. The character of the basal shape of vein R2+3 seems to be the most exclusive external character to separate the subfamily from other Syrphidae. A key to distinguish Microdontinae from other Syrphidae is given below. As not all Syrphidae have been studied, doubtful cases may occur, so it is recommended to verify at least a few of the other characters in Table 2, preferably those of the male genitalia.
Characters considered to be of good diagnostic value for separating Microdontinae from other Syrphidae, with indication of known exceptions. See text for Discussion.
| Character statement | State in Microdontinae | Exceptions | State in other Syrphidae | Exceptions |
|---|---|---|---|---|
| Head | ||||
| eyes of male, contiguity | dichoptic | Spheginobaccha chillcotti | usually holoptic | several |
| Thorax | ||||
| postpronotum, pilosity | present | several, e.g. Masarygus, Surimyia, Paramixogaster | Syrphinae: bare<br/> Eristalinae: pilose | a few Syrphinae (e.g. Allobaccha Curran, 1928) |
| postmetacoxal bridge, presence | present | Rhoga (in part), Spheginobaccha | absent | e.g. Baccha elongata (Fabricius, 1775), Leucopodella Hull, 1949, Neoascia, Sphegina |
| plumule, degree of development | short or absent | none | long | Cerioidini, Neoascia, Sphegina, Eosphaerophoria Frey, 1946, Allograpta ventralis (Miller, 1921), Ocyptamus Macquart, 1834 in part. |
| Wing | ||||
| stigmal crossvein, presence | present | Paramicrodon delicatulus, Spheginobaccha | Syrphinae: absent<br/> Eristalinae: variable | unknown |
| vein R2+3, shape basal part | strongly curved (Fig. 435: angle A > angle B) | none | weakly curved (Fig. 435: angle A < angle B) | Cerioidini |
| Legs | ||||
| femora and tibiae, presence of subbasal and subdistal cicatrices | present | Masarygus palmipalpus, Piruwa phaecada, Schizoceratomyia flavipes | absent or weakly developed | none |
| Abdomen | ||||
| abdomen, number of preabdominal segments | four | none | Syrphinae: five, except four in Pipizini<br/> Eristalinae: four | none |
| Male genitalia | ||||
| postgonites, presence | absent | none | present | none |
| phallus, point of articulation with hypandrium | basal | Spheginobaccha guttula | apical | none |
| phallus, apical part, shape | tubular, elongate, without separate structures (often furcate) | none | rarely elongate, usually with separate structures | |
| phallus, basal part, shape | usually spherical | Archimicrodon | never spherical | none |
| phallapodeme, presence | absent | Spheginobaccha (African taxa only) | present | none |
| Character statement | State in Microdontinae | Exceptions | State in other Syrphidae | Exceptions |
|---|---|---|---|---|
| Head | ||||
| eyes of male, contiguity | dichoptic | Spheginobaccha chillcotti | usually holoptic | several |
| Thorax | ||||
| postpronotum, pilosity | present | several, e.g. Masarygus, Surimyia, Paramixogaster | Syrphinae: bare<br/> Eristalinae: pilose | a few Syrphinae (e.g. Allobaccha Curran, 1928) |
| postmetacoxal bridge, presence | present | Rhoga (in part), Spheginobaccha | absent | e.g. Baccha elongata (Fabricius, 1775), Leucopodella Hull, 1949, Neoascia, Sphegina |
| plumule, degree of development | short or absent | none | long | Cerioidini, Neoascia, Sphegina, Eosphaerophoria Frey, 1946, Allograpta ventralis (Miller, 1921), Ocyptamus Macquart, 1834 in part. |
| Wing | ||||
| stigmal crossvein, presence | present | Paramicrodon delicatulus, Spheginobaccha | Syrphinae: absent<br/> Eristalinae: variable | unknown |
| vein R2+3, shape basal part | strongly curved (Fig. 435: angle A > angle B) | none | weakly curved (Fig. 435: angle A < angle B) | Cerioidini |
| Legs | ||||
| femora and tibiae, presence of subbasal and subdistal cicatrices | present | Masarygus palmipalpus, Piruwa phaecada, Schizoceratomyia flavipes | absent or weakly developed | none |
| Abdomen | ||||
| abdomen, number of preabdominal segments | four | none | Syrphinae: five, except four in Pipizini<br/> Eristalinae: four | none |
| Male genitalia | ||||
| postgonites, presence | absent | none | present | none |
| phallus, point of articulation with hypandrium | basal | Spheginobaccha guttula | apical | none |
| phallus, apical part, shape | tubular, elongate, without separate structures (often furcate) | none | rarely elongate, usually with separate structures | |
| phallus, basal part, shape | usually spherical | Archimicrodon | never spherical | none |
| phallapodeme, presence | absent | Spheginobaccha (African taxa only) | present | none |
| 1 | Vein R2+3 weakly curved basally: angle A < angle B (Fig. 435) | Syrphinae and Eristalinae (ex. Cerioidini) |
| – | Vein R2+3 strongly curved basally: angle A > angle B (Fig. 435) | 2 |
| 2 | Antenna with terminal arista. Male holoptic. | Eristalinae (Cerioidini) |
| – | Antenna with dorsal arista, or without arista. Male dichoptic | Microdontinae |
| 1 | Vein R2+3 weakly curved basally: angle A < angle B (Fig. 435) | Syrphinae and Eristalinae (ex. Cerioidini) |
| – | Vein R2+3 strongly curved basally: angle A > angle B (Fig. 435) | 2 |
| 2 | Antenna with terminal arista. Male holoptic. | Eristalinae (Cerioidini) |
| – | Antenna with dorsal arista, or without arista. Male dichoptic | Microdontinae |
The first author is grateful to Dr. Kees van Achterberg for allowing him to use his microscopic and photographic facilities on many, many occasions. The following collection managers and research entomologists kindly provided assistance in various ways, e.g. during visits, with arranging loans or by sending material from their personal collections: Stephan Blank (DEI), Brian Brown, Ben Brugge (ZMAN), Daniel Burckhardt (NMB), Rune Bygebjerg (MZLU), Christophe Daugeron (MNHN), E. De Coninck (RMCA), Mr. Delfosse (MNHN), Marc De Meyer (RMCA), Steve Gaimari (CSCA), Aniel Gangadin (NZCS), David Grimaldi (AMNH), Patrick Grootaert (KBIN), Peter Haase (SMF), Martin Hauser, Richard Hoebeke (CU), Niklas Jönsson (NHRS), Uwe Kallweit (SNSD), Chris Manchester (ANIC), Luciane Marinoni (UFPR), Ximo Mengual, Frank Menzel (DEI), Y. Nakatani (NIAS), Tam Nguyen (AMNH), Mirian Nunes Morales (UFPR), Burgert Muller (NMSA), Thomas Pape (ZMUC), Philip Perkins (MCZ), Chris Raper, Peter Sehnal (NMW), Jeff Skevington (CNC), Zoë Simmons (OUMNH), John T. Smit, Daniele Sommaggio, Chris Thompson (USNM), Wouter van Steenis, Rob de Vries (RMNH), Nigel Dr. Meicai Wei (CSCS), Shaun Winterton, Nigel Wyatt (BMNH), David Yeates (ANIC), S. Yoshimatsu (NIAS), Chen Young (CM), Joachim Ziegler (ZMHU), Manuel Zumbado (INBIO).
Last but foremost, we want to thank F. Christian Thompson for his many advices and generous sharing of information, and for encouraging the first author to work on the Microdontinae, a group on which Chris has done so much invaluable work both recently and in previous decades.
This section contains descriptions of 26 previously undescribed species, and a redescription of Ceratrichomyia behara Séguy, 1951. Most of these were included in the phylogenetic analyses of Reemer and Ståhls (in press). In addition, some new species are described which were considered interesting for other reasons, for instance because they considerably extend the known range of a genus (e.g. Ceratrichomyia from mainland Africa, Kryptopyga from Sulawesi). Ceratrichomyia behara Séguy, 1951 is redescribed, because the type series was found to contain three different species (see genus account in previous section). Characters additional to those mentioned in the descriptions can be found in the morphological character matrix of Reemer and Ståhls (in press).
Line breaks on the original specimen labels are indicated with a slash (/).
As in all other known Microdontinae except Spheginobaccha chillcotti Thompson, 1974, the males are dichoptic (i.e. eyes not touching each other dorsally).
urn:lsid:zoobank.org:act:385134E2-9482-409E-AC2D-D432E8315FE4
http://species-id.net/wiki/Archimicrodon_malukensis
Figs 10–15HOLOTYPE. Adult male. INDONESIA. Label 1: “INDONESIA: HALMAHEIRA / near Payake. 115 m. / Mal. trap. 18.II-18.III.1995 / C. v. Achterberg & R. de Vries”. Coll. RMNH.
PARATYPES. One male and one female from same locality and date as holotype. One male from Halmaheira, near Akeiamo, alt. 175 m., 18.II-18.III.1995, leg. C. van Achterberg & R. de Vries, coll. RMNH (this specimen was used in the morphological matrix of Reemer and Ståhls in press; voucher code MR124).
The entirely black head, thorax (including femora and tibiae) and abdomen (whether or not with metallic hues) are shared with five other described Archimicrodon-species of the Indo-Australian region (Australia excluded). Archimicrodon boharti (Curran, 1947) (Solomon Islands) differs from this species by the metallic blue shining scutellum, clearly contrasting with the non-metallic mesonotum (in Archimicrodon malukensis sp. n. mesonotum and scutellum are of the same black colour). The same character also applies to Archimicrodon limbinervis (de Meijere, 1908) and Archimicrodon incisuralis (Walker, 1865) from New Guinea, and Archimicrodon purpurescens (Shiraki, 1963) from Micronesia, which also differ by the black pilose scutellum (white pilose in Archimicrodon malukensis). Archimicrodon grageti (de Meijere, 1908) (New Guinea) differs by the brownish abdomen and reddish yellow pregenital segments (black in Archimicrodon malukensis).
Adult male. Body size: 8 mm.
Head. Face occupying about 1/5 of head width in frontal view; black; black pilose, except white pilose on ventral 1/4. Gena hardly developed; black; white pilose. Oral margin not produced. Frons black; black pilose, except white pilose along lateral margin. Vertex black; black pilose. Occiput black; black pilose dorsally, white pilose ventrally. Eye bare. Antennal fossa about as high as wide. Antenna black; antennal ratio approximately as 2:1:3.
Thorax. Thorax black, except pleurae brownish. Mesoscutum black pilose, except pale yellow pilose along anterior margin, laterally between postpronotum and notopleuron, and in posterolateral corners. Postpronotum pale yellow pilose. Postalar callus black pilose, except pale yellow pilose at posterior apex. Scutellum semicircular, without calcars; entirely pale yellow pilose. Anepisternum with shallow dorsomedian sulcus; white pilose anterodorsally and posterodorsally, widely bare in between. Anterior anepimeron entirely white pilose. Katepimeron sparsely white pilose along dorsal margin, otherwise bare. Katatergum long microtrichose, anatergum short microtrichose. Calypter and halter pale yellow.
Wing: Hyaline, slightly infuscated antero-apically; microtrichose, except bare on cell sc, basal 1/2 of cell c, basal 2/5 of cell r1, most of cell br except microtrichose along vena spuria, posterobasal 1/5 of cell r4+5, basal 5/6 of cell bm, anterobasal 3/5 of cell cup, basomedian 2/3 of alula and basal 1/6 of anal lobe.
Legs: Black, except fifth tarsomeres brown; black pilose, except femora posterobasally white pilose and tarsi ventrally golden yellow pilose. Coxae black; white pilose. Trochanters brown; white pilose.
Abdomen. Tergites black with faint metallic hues, except for a dull black fascia on anterior 2/5 of tergite 3 and a very narrow, medially interrupted dull black fascia along anterior margin of tergite 4. Tergites 1 and 2 yellowish white pilose. Tergites 3 and 4 black pilose, except white pilose posterolaterally. Sternites blackish brown; sternite 1 bare; sternite 2 yellow pilose; sternite 3 black pilose except yellow pilose along posterior margin; sternite 4 black pilose. Male genitalia as in Fig. 15.
Female. 9.5 mm. As male, except for usual sexual differences. Tergite 5 black pilose, except white pilose posteriolaterally.
The specific epithet (adjective) is derived from Maluku, the group of islands to which Halmaheira, where the species was found, belongs.
urn:lsid:zoobank.org:act:7F7925F3-C102-432C-A1C4-167902226596
http://species-id.net/wiki/Ceratrichomyia_angolensis
Figs 51–56HOLOTYPE. Male. Label 1: “ANGOLA 30 km NE / Duque de Bragan - / za, Nov. / Dec. 1957”; label 2: “Collector / G.H. Heinrich”. Coll. CNC.
This species differs from both other known species of Ceratrichomyia by the bare postpronotum and katepimeron, the downward projecting face, and the absence of a ventrolateral ridge on the epandrium.
Adult male. Body size: 10 mm.
Head. Face occupying approximately 1/2 of head width in frontal view; yellowish brown, except for blackish marks dorsally along eye margin; entirely yellow pilose; with pit-like depressions on dorsal 1/3; face profile more or less straight, strongly produced ventrally below eye margin. Gena yellowish brown. Lateral oral margins not produced. Frons and vertex yellowish brown, a little blackish at and around ocellar triangle; yellow pilose. Occiput yellow; dorsally wide and yellow pilose, ventrally narrow and whitish pilose. Eye bare. Antennal fossa about as wide as high. Antenna orange brown, except basoflagellomere blackish brown; antennal ratio approximately as 6:1:18. Basoflagellomere very long, entirely covered with pile at least as long as 1.5 times diameter of basoflagellomere. Arista very small, shorter than pedicel; situated at about 1/3 from base of basoflagellomere.
Thorax. Mesonotum dorsally black, with margins widely yellowish brown, and with pair of narrow yellow submedian vittae, dividing black into three approximately equally wide parts; short, appressed yellow pilose; transverse suture yellowish brown. Postpronotum yellowish brown; bare. Postalar callus yellowish brown; yellowish pilose. Scutellum blackish brown; yellow pilose; without calcars. Pleurae orange brown. Anepisternum with deep sulcus separating anterior and posterior part; entirely yellow pilose. Anepimeron entirely yellow pilose. Katepisternum yellow pilose dorsally and ventrally. Katepimeron bare. Katatergum with short microtrichia, anatergum bare. Calypter yellowish brown. Halter yellow.
Wing: hyaline; microtrichose, except bare on cells bc and c, basal 1/2 of cell r1, almost entirely on cells br, bm, cup and on alula, and posterobasal 1/5 of cell r4+5. Vein bm-cu shorter than basal section of CuA1.
Legs: Orange brown except femora blackish brown on basal 1/2; yellow pilose. Coxae and trochanters blackish brown; pale pilose.
Abdomen. Constricted at 2nd segment, with narrowest point at posterior margin, widest halfway of tergite 4 (slightly wider than thorax). Tergites 1-3 fused. Tergite 1 dark brown; yellow pilose. Tergite 2 pale yellow; yellow pilose along lateral margin. Tergites 3 and 4 dark brown; yellow pilose. Sternite 1 yellow; bare. Sternite 2 yellow anteriorly, brown posteriorly; mixed yellow and black pilose. Sternite 3 dark brown; black pilose. Sternite 4 concealed behind genital capsule; brown; yellow pilose. Genitalia as in Fig. 56.
Female unknown.
The specific epithet (adjective) is derived from Angola, where the species was found.
http://species-id.net/wiki/Ceratrichomyia_behara
Figs 46, 47, 57LECTOTYPE. Male. Label 1: “Madagascar, Behara”; label 2 (blue): “Museum Paris, III-38, A. Seyrig”; label 3 (red): “Type”; label 4: “Ceratrichomyia behara type du genre [male symbol] Séguy 50”. Coll. MNHN. See genus account of Ceratrichomyia for notes on lectotype designation.
This species differs from Ceratrichomyia angolensis sp. n. by the pilose postpronotum and katepimeron. From Ceratrichomyia bullabucca sp. n. it differs by the straight face profile and the parallel-sided tergite 2.
Adult male. Body size: 7 mm.
Head. Face occupying approximately 1/2 of head width in frontal view; yellow; entirely yellowish white pilose; depressed on lateral 1/3; face profile more or less straight. Gena yellow. Lateral oral margins not produced. Frons and vertex yellow; yellow pilose. Occiput yellow; dorsally wide and yellow pilose, ventrally narrow and whitish pilose. Eye bare. Antennal fossa about as wide as high. Antenna orange brown, getting dark brown towards apex of basoflagellomere; antennal ratio approximately as 1:0, 2:3, 5. Basoflagellomere very long, entirely covered with pile at least twice as long as diameter of basoflagellomere. Arista very small, shorter than pedicel.
Thorax. Mesoscutum, postpronotum, postalar callus and scutellum reddish brown; short, yellow pilose. Scutellum without calcars. Pleurae orange brown. Anepisternum with deep sulcus separating anterior and posterior part; entirely whitish pilose. Anepimeron entirely pale pilose. Katepisternum densely white pilose dorsally; sparsely pilose ventrally. Katepimeron white pilose. Katatergum with long microtrichia, arranged in oblique rows. Anatergum short microtrichose. Calypter brownish. Halter yellow.
Wing: hyaline; microtrichose, except bare on cell bc, basally on cell r1 along vein Rs, on most of cell br except microtrichose along vena spuria, on most of cell bm except apical 1/8, basal 1/2 of cell cup. Vein bm-cu shorter than basal section of CuA1.
Legs: Orange except femora blackish with orange apical 1/4; pale pilose, except tarsi dorsally black pilose. Coxae and trochanters blackish brown; pale pilose.
Abdomen. Constricted at 2nd segment, with tergite 2 parallel-sided, widest at tergite 3 and 4 (slightly wider than thorax). Tergite 1 dark brown; white pilose. Tergite 2 dorsoventrally flattened, dark brown with large, triangular yellow maculae along lateral margin, posteriorly interconnected and reaching posterior margin, which is entirely yellow; white pilose. Tergite 3 and 4 dark brown with yellow posterior margins; white to yellow pilose. Tergite 4 with two faint submedian grooves from anterior margin to just before posterior margin. Sternite 1 yellow; bare. Other sternites brown; white pilose. Genitalia as in Fig. 57.
Female. Unknown.
urn:lsid:zoobank.org:act:2B7970AE-82E2-45A6-B496-213889A5EA60
http://species-id.net/wiki/Ceratrichomyia_bullabucca
Figs 48–50, 58HOLOTYPE [this specimen is one of the paratypes of Ceratrichomyia behara Séguy]. Male. Label 1: “Madagascar, Bekily, Rég. sud de l’ile”; label 2 (blue): “Museum Paris, X.36, A. Seyrig”; label 3: “Ceratrichomyia behara cotype, male, Séguy 1950”. Coll. MNHN.
This species differs from Ceratrichomyia angolensis sp. n. by the pilose postpronotum and katepimeron. From Ceratrichomyia behara Séguy, 1951 it differs by the convex face profile and the anteriorly widened tergite 2.
Adult male. Body size: 8.5 mm. As Ceratrichomyia behara, except for differences listed below.
Head. Face occupying almost 3/5 of head width in frontal view. Face profile clearly convex.
Thorax. Katepisternum bare ventrally. Calypter yellow.
Wing: cell r1 entirely microtrichose, cell br bare on posterobasal 3/5. Vein bm-cu longer than basal section of CuA1. Abdomen. Tergite 2 not parallel-sided: narrowest point at about half its length; lateral yellow macula, not connected posteriorly. Genitalia as in Fig. 58.
Female. Unknown.
The specific epithet (noun in apposition) contains the Latin words bulla (bubble, knob) and bucca (cheek) and refers to the swollen face, a character to distinguish the species from Ceratrichomyia behara.
urn:lsid:zoobank.org:act:78CD4D02-D4C0-434D-ACDE-1A9D214DD9B2
http://species-id.net/wiki/Domodon_zodiacus
Figs 68–73HOLOTYPE. Male, SURINAM, Paramaribo Zoo, 05°50'30"N, 55°09'29"W, malaise trap, 18-27.II.2006, leg. M. Reemer. Coll. RMNH.
Three undescribed species belonging to this genus are known. From those, Domodon zodiacus sp. n. can be distinguished by the following combination of characters: face with black median vitta, alula entirely microtrichose, tergites 3 and 4 partly yellow.
Adult male. Body size: 7 mm.
Head. Dichoptic. Face occupying about 1/3 of total head width in frontal view; pale yellow with brown median vitta of 1/5 of facial width; entirely yellow pilose; not pollinose; eye margins slightly converging at level of frons, with smallest distance approximately equal to three times width of antennal fossa. Gena black. Oral margin laterally produced; black. Antennal fossa about as wide as high. Frons black with metallic green shine; golden pilose. Vertex convexly produced; shining black; sparsely short pilose. Ocellar triangle not elevated; anterior angle about 100°. Occiput narrow; black; golden yellow pilose dorsally, white pilose ventrally. Eye bare. Antenna dark brown; antennal ratio approximately 4:1:4; basoflagellomere elongate with rounded apex, with small sensory pit located at about 1/3 from base; arista slender, about 2/3 of length of basoflagellomere.
Thorax. Mesoscutum black with faint metallic hues; black pilose, except for a narrow sutural and a wide prescutellar fascia of golden pilosity. Postpronotum blackish; yellow pilose. Postalar callus brown; yellow pilose. Scutellum with two apical calcars of 1/4 of length of scutellum; brown with faint metallic hues. Pleurae blackish brown. Anepisternum with anterior and posterior part separated by clear sulcus; anterior part short black pilose, posterior part long yellow pilose, with bare area in between. Anterior anepimeron entirely pale yellow pilose. Katepisternum yellow pilose dorsally, bare ventrally. Katatergum with long black microtrichia. Anatergum short pale microtrichose. Other pleurites bare. Calypter and halter yellow.
Wing: Hyaline, faintly darker around crossvein r-m; microtrichose, except bare on cell bc, posterobasal 1/2 of cell c, basally on cell r1 along vein Rs, on cell br except along vena spuria and extreme apex, on posterobasal 1/2 of cell bm, on anterobasal 1/2 of cell cup.
Legs: Anterior four legs pale brown, with vaguely defined darker and paler parts; femora black pilose except mid-femur pale pilose posteriorly; tibiae pale pilose dorsally, black pilose ventrally; tarsi black pilose except last tarsomere yellow pilose. Hind femur blackish with apical 1/3 yellow; black pilose anteriorly, pale pilose posteriorly. Hind tibia dark brown with pale apex; black pilose dorsally, pale pilose ventrally. Hind tarsus brown with last tarsomere yellowish; black pilose, except last tarsomere yellow pilose.
Abdomen. Ratio of median tergal lengths approximately as 1:2:3:5. Tergites 3 and 4 not clearly fused, only laterally. Tergite 1 black; pale pilose. Tergite 2 pale yellow with lateral 1/4 black and with posteriomedian black macula; yellow parts yellow pilose, black parts black pilose. Tergite 3 pale yellow with extreme lateral margins black, with sublateral oblique black maculae of slightly less than 1/3 of tergal width, with narrow median black vitta on anterior 2/3; black pilose except yellow pilose along posterior margin. Tergite 4 black except yellow along lateral and posterior margins; black pilose except yellow pilose on yellow parts. Sternite 1 black; bare. Other sternites yellow, sparsely pilose. Genitalia as in 84.
Female. Unknown.
The name zodiacus (adjective, Greek: ‘of animals’) was chosen because the type specimen was collected at the Paramaribo Zoo.
urn:lsid:zoobank.org:act:603D5305-E43A-4BDD-90F6-CE796366D40C
http://species-id.net/wiki/Furcantenna_nepalensis
Figs 74–80HOLOTYPE. Adult male. NEPAL. Label 1: “NEPAL, Ktmd. / Godavari 6000’ / 13 Aug. 1967 / Can. Nepal Exped.”. Coll. CNC.
Three characters are mentioned by Cheng and Thompson (2008) to distinguish Furcantenna Cheng, 2008 from the Neotropical genus Schizoceratomyia Carrera, Lopes & Lane, 1947: scutellum apicomedially sulcate, katepisternum pilose, metasternum developed and pilose. All three characters are found in the species described here. Only one other species of Furcantenna is known (Furcantenna yangi Cheng, 2008). From that species, Furcantenna nepalensis sp. n. differs by the following characters (characters of Furcantenna yangi in parentheses, based on Cheng and Thompson 2008): body colour brownish, without violet shine (black, with violet shine); mesoscutum entirely golden pilose (black and white pilose); katepimeron pilose (bare); tergite 2 with ratio of median length : width of posterior margin approximately as 1:3 (1:6).
Adult male. Body size: 10 mm.
Head. Blackish brown. Face occupying about 1/2 of head width in frontal view; laterally depressed and dull, medially with shining carina; white pilose. Gena white pilose. Oral margin not produced. Frons, vertex and occiput golden pilose. Eye bare. Antennal fossa slightly higher than wide. Antenna: scape pale brown, pedicel and basoflagellomere black; antennal ratio approximately as 5:1:17.5; basoflagellomere bifurcate at base, both branches entirely long pilose; arista absent.
Thorax. Mesoscutum black, except pale brown along margins; golden pilose. Postpronotum and postalar callus brown; golden pilose. Scutellum trapezoid, although slightly sulcate apicomedially; without calcars; brown; golden pilose. Pleurae brown. Anepisternum with deep median sulcus; golden pilose, except bare on ventral 1/5. Anterior anepimeron entirely golden pilose. Katepisternum white pilose dorsally, bare ventrally. Katepimeron white pilose. Katatergum long microtrichose, anatergum short microtrichose. Calypter and halter pale yellow.
Wing: Hyaline, tinged with brown, especially on anterior half. Microtrichose, except bare on basal 2/3 of cell bc, posterobasal 1/2 of cell br, and anterobasal 1/2 of cell bm.
Legs: Brown. Front leg golden yellow pilose, except tarsus dorsally black pilose. Mid and hind legs black pilose, except femora largely golden yellow pilose and tarsi ventrally golden pilose. Coxae and trochanters brown; whitish pilose.
Abdomen. Tergites dark brown, a little paler along lateral margins and entirely on tergite 4. Tergite 1 yellow pilose. Tergites 2 and 3 golden yellow pilose anteriorly and laterally, black pilose medially and posteriorly. Tergite 4 entirely golden pilose. Sternites dark brown; all sternites yellowish white pilose. Male genitalia as in Fig. 80.
Female. Unknown.
The name nepalensis (noun in the genitive case) refers to the type locality.
urn:lsid:zoobank.org:act:264A9CEC-A0C4-44AE-8160-7D44E7C8DACD
http://species-id.net/wiki/Heliodon_doris
Figs 81–87, 94HOLOTYPE. Adult male. THAILAND. Label 1: “THAILAND Ubon Ratchathani, Pha Taem / NP, west of Huay Pok substation, 438 m, / Malaise trap, 15°37.212'N, 105°36.903'E, / 25.iv–2.v.2007, Bunlu Sapsiri leg. T2173”; label 2: “Voucher code M. Reemer / 314 / DNA voucher labcode MZH:Y1074”. Coll. QSBG.
PARATYPES. THAILAND: Adult female; label 1: “THAILAND, Loei, T485 / Phu Kradueng NP, Malaise / 16°51.958'N, 101°50.668'E / 16.VIII-23.VIII.2006. 280 m / Sutin Khonglasae leg.”; coll. RMNH. MALAYSIA: Adult male; label 1: “G.6722 / Malaya / Selangor / F.E.S. Serdang / 22.3.1956”; label 2: “COM INST ENT. / COLL NO 14927”; label 3: “Microdon / [female sign] not in B.M. / van Emdendet. 1956”; label 4: “Pres. by / Com. Inst. Ent. / B.M. 1956-712”; label 5: “Microdontini / ? new genus / N.P. Wyatt det. 1985”. Coll. BMNH.
Within Heliodon, no other species has tergites 3 and 4 predominantly yellow.
Adult male. Body size: 9 mm.
Head. Face occupying slightly more than 1/4 of head width in frontal view; yellow; yellow pilose. Gena yellow; yellow pilose. Lateral oral margins weakly produced. Frons blackish brown; golden pilose. Vertex blackish; golden pilose. Occiput black; golden yellow pilose dorsally, whitish yellow pilose ventrally. Eye bare. Antennal fossa about as high as wide. Antenna brown, except scape and pedicel yellow ventrally; antennal ratio approximately as 3.5:1:2.5.
Thorax. Mesoscutum black with metallic hues, except yellow around postpronotum, anteriad of notopleuron and around postalar callus; golden pilose. Postpronotum, postalar callus and scutellum yellow; golden pilose. Scutellum semicircular; with pair of apical calcars with mutual distance slightly larger than length of scutellum. Pleurae yellow, except anepisternum dark brown along anterior margin, katepisternum dark brown ventrally, meron and metanotum dark brown; all pilosity golden yellow. Anepisternum enitrely pilose, except narrowly bare along ventral margin; with shallow sulcus separating anterior from posterior part. Anepimeron entirely pilose. Katepisternum pilose dorsally, bare ventrally. Katatergum long microtrichose, anatergum short microtrichose. Calypter and halter pale yellow.
Wing: Hyaline, slightly darkened around apical crossveins; microtrichose, except bare on cell sc, basal 3/4 of cell c, basal 1/3 of cell r1, entirely on cell br except microtrichose along vena spuria, basal 1/4 of cell r4+5, basal 3/4 of cell bm, anterobasal 3/4 of cell cup and basomedian 1/2 of alula.
Legs: Front leg yellow; yellow pilose. Other legs missing in holotype. [Paratype male: mid leg yellow, yellow pilose; hind femur dark brown except yellow on basal 1/4 and apical 1/10, yellow pilose; hind tibia yellow on basal 3/5, dark brown on apical 2/5, yellow pilose; hind tarsus with first tarsomere dark brown dorsally, otherwise yellow, yellow pilose.] Front and mid coxae yellow; yellow pilose. Hind coxa dark brown basally, yellow apically; yellow pilose. Trochanters yellow; yellow pilose.
Abdomen. Slightly constricted, with narrowest point at posterior margin of tergite 2. Tergite 1 dark brown; yellow pilose. Tergite 2 yellow with dark brown, triangular median macula, with narrowest part at anterior margin and widest part close to posterior margin; yellow pilose, except dark pilose laterally. Tergite 3 yellow with median dark brown vitta and pair of oblique, lateral, dark brown maculae; yellow pilose, except dark pilose on and around lateral dark maculae. Tergite 4 with colour pattern similar to tergite 3, but lateral maculae anteriorly confluent with median vitta; golden yellow pilose, except dark pilose on lateral maculae. Sternites yellow, slightly darkened on sternite 1, 3 and 4; yellow pilose. Male genitalia as in Fig. 94.
Female. As male, except for following differences. Dark maculae on tergite 4 not confluent anteriorly. Tergite 5 yellow with dark brown median vitta and pair of small, round, submedian dark brown spots; golden yellow pilose, except for black pile anteromedially and sublaterally. Sternite 3 dark brown medially. Sternites 4 and 5 dark brown.
This species is named after my daughter Doris. The epithet is a noun in apposition.
urn:lsid:zoobank.org:act:B6CB7238-C92A-4641-B824-EE738907F179
http://species-id.net/wiki/Heliodon_elisabethanna
Figs 88–90HOLOTYPE. Adult female. THAILAND. Label 1: “THAILAND / 2007”; label 2: “Voucher code M. Reemer / 316 / DNA voucher labcode MZH:Y1062”. Coll. QSBG. No further locality data available.
No other species of Heliodon has entirely black legs.
Adult female. Body size: 12 mm.
Head. Face occupying about 1/3 of head width in frontal view; black; entirely golden pilose. Gena black; golden pilose. Lateral oral margins produced. Frons black; black pilose, except golden pilose posterolaterally. Vertex black; black pilose, except golden pilose along anterior margin and white pilose along posterior margin. Occiput black; black pilose on dorsal 1/3, white pilose on ventral 2/3. Eye pale pilose, with pile approximately as long as half the diameter of frontal ocellus. Antennal fossa about as high as wide. Antenna brown; antennal ratio approximately as 3:1:2.5.
Thorax. Mesoscutum black; black pilose, with inconspicuous pale pile along anterior margin and along lateral 1/3 of transverse suture. Postpronotum brownish; pale pilose. Postalar callus black on anterior 3/4, brown on posterior 1/4; black pilose dorsally, pale pilose laterally. Scutellum semicircular; with pair of distinct apical calcars with mutual distance about 1/3 of width of scutellum at base; black; black pilose anteriorly and dorsally, long golden pilose posteriorly. Pleurae black. Anepisternum black pilose, except white pilose on ventral 1/3 and narrowly along anterior margin; with very small bare patch ventrally on anterior part; with deep sulcus separating anterior from posterior part. Anepimeron black pilose on dorsal 1/3, white pilose on ventral 2/3. Katepisternum white pilose dorsally, bare ventrally. Katatergum long microtrichose, anatergum short microtrichose. Calypter greyish. Halter white.
Wing: Hyaline, except vaguely infuscated around apical crossveins, around spur on vein R4+5, around base of R2+3 and crossvein r-m; microtrichose, except bare on cell bc, basal 1/2 of cell c, basally on cell r1 along vein Rs, entirely bare on cell br except microtrichose along vena spuria, on basal 1/2 of cell r4+5, basal 1/2 of cell bm, basal 1/3 of cell cup, basomedian 2/3 of alula.
Legs: Black, except front femur brown anteriorly; white pilose, except golden pilose basoventrally on front and mid femora and apicoventrally on front and mid tibiae, and golden pilose ventrally on tarsi. Coxae and trochanters black; white pilose.
Abdomen. Tergites black, except for large yellow maculae posterolaterally on tergite 2, and narrow, medially interrupted yellow fasciae along posterior margins of tergites 3 and 4. Tergite 1 inconspicuously pale pilose. Tergite 2 with thick, conspicuous, appressed golden pile (tomentum), except narrowly black pilose along anterior margin. Tergite 3 with medially interrupted fascia of golden tomentum on posterior 2/5, inconspicuous golden pile on lateral 1/3 and inconspicuous black pile anteriorly. Tergite 4 with medially interrupted fascia of golden tomentum on posterior 1/2, inconspicuous golden pile on lateral 1/4 and inconspicuous black pile anteriorly. Tergite 5 medially with pair of large, medially connected patches of golden tomentum, mixed black and golden pilose otherwise. Sternites brownish; sternites 1-3 yellowish pilose; sternites 4 and 5 black pilose.
Female. Unknown.
This species is named after my partner Elisabeth (Liesbeth) Anna. The epithet is a noun in apposition.
urn:lsid:zoobank.org:act:E3CFF355-9DA9-42C1-B41E-17A873962AA0
http://species-id.net/wiki/Heliodon_tiber
Figs 91–93, 95HOLOTYPE. Adult male. INDONESIA (Sumatra). Label 1: “Fort de Kock / (Sumatra) 920 M. / 1924 / leg. E. Jacobson”. Coll. ZMAN.
PARATYPE. Adult male. INDONESIA (Sumatra). Label 1: “Fort de Kock / (Sumatra) 920 M. / 1925 / leg. E. Jacobson”; label 2: “Microdon / fascipennis / Sack”. Coll. ZMAN.
This is the only known species of Heliodon in which the hind femur is entirely yellow.
MALAYSIA: 2 males, Selangor, Gombak Field Stn., 14.XI.1977, leg. B. Bendell; 1 male, Pahang, Frazer’s Hill, 27.X-3.XI.1977, leg. B. Bendell. THAILAND: 1 female, Loei, T1108, Phu Ruea NP, 17°29.652'N, 101°21.020'E, 1167 m., 5-6.xi.2006, pan trap, leg. Patikhom Tumtip, coll. M. Hauser; 1 female, Phetchabun, Nam Nao NP, Heliport, 16°43.156"N, 101°35.118"E, 890 m., 18-25.xii.2006, leg. Noopean Hongyothi, coll. RMNH. VIETNAM: 1 female, Chu Yang Sin Nat. Park, 1-10.VI.2007, mal. trap, leg. C. van Achterberg & R. de Vries; DNA voucher labcode MZH:Y1072, in coll. RMNH.
Adult male. Body size: 12 mm (paratype 10 mm).
Head. Face occupying about 1/3 of head width in frontal view; black, except brownish yellow on lateral 1/6; entirely white pilose. Gena black; white pilose. Lateral oral margins weakly produced. Frons and vertex black; white pilose. Occiput black; white pilose. Eye pilose, with pile approximately as long as diameter of ocelli. Antennal fossa about as high as wide. Antenna brown; antennal ratio approximately as 3.5:1:2.
Thorax. Mesoscutum black; yellow pilose, with pile thicker and more appressed along anterior margin, along transverse suture and along posterior margin, forming three transverse fasciae. Postpronotum and postalar callus brown; yellow pilose. Scutellum semicircular; with pair of distinct apical calcars with mutual distance about 1/4 of width of scutellum at base; brown; yellow pilose. Pleurae shining black; all pilosity yellowish white. Anepisternum pilose, except anterior part bare ventromedially; with deep sulcus separating anterior from posterior part. Anepimeron entirely pilose. Katepisternum pilose dorsally, bare ventrally. Katatergum long microtrichose, anatergum short microtrichose. Calypter and halter pale yellow.
Wing: Hyaline, except infuscated around apical crossveins, around spur on vein R4+5 and around base of R2+3, crossvein r-m and bm-cu; microtrichose, except bare on cell bc, posterobasal 1/10 of cell c, basally on cell r1 along vein Rs, basal 3/4 of cell br, anterobasal 1/5 of cell cup, basomedian 2/3 of alula.
Legs: Brownish yellow, with tibiae slightly infuscated medially; entirely yellow pilose. Coxae blackish brown; yellow pilose. Trochanters yellow; yellow pilose.
Abdomen. Tergites dark brown, except tergites 2-3 yellow laterally. Tergite 1 yellowish pilose. Tergite 2 yellowish pilose, except silvery white pilose along posterior margin. Tergite 3 silvery white pilose along anterior and posterior margins, yellow pilose along lateral margins, black pilose medially. Tergite 4 silvery white pilose along anterior margin and on posterior 1/2, black pilose medially. Sternites brown. Sternite 1 and 2 white pilose, sternite 3 and 4 black pilose. Male genitalia as in Fig. 95.
Female. As male, except for following differences. Body size 8–12 mm. Overall colouration paler: whereas pale parts are brownish in the examined males, these parts are yellowish in the examined females. The scutellar spines are less strongly developed, and in one of the examined females from Thailand even totally absent.
This species is named after my son Tiber. The epithet is a noun in apposition.
In the paratype and in all additionally studied specimens, the pilosity of thorax and abdomen is more golden yellow than in the holotype, also in the parts which are silvery white in the holotype. In most specimens the legs are entirely yellow, without infuscated parts.
The paratype has a label stating “Microdon fascipennis Sack” (or possibly fuscipennis) in what seems to be the handwriting of J.C.H. De Meijere (judged by comparison with figures of de Meijere’s handwriting in De Jong 2000). However, no such name is known to have been given to any Microdontinae, neither by Sack nor by any other author. Either De Meijere was mistaken, or the name is an unpublished manuscript name.
urn:lsid:zoobank.org:act:F26FF6CC-E819-4A7B-AFA3-9E7DB82C004B
http://species-id.net/wiki/Indascia_gigantica
Figs 107–112HOLOTYPE. Adult male. THAILAND. Label 1: “THAILAND: Chiang Mai, Doi Inthanon NP / Checkpoint 2, 18°31.554'N, 98°29.94'E 1700 m / Malaise trap 8-15.v.2007, Y. Areeluck leg. T1832”; label 2: “Syrphidae / T1832 / W. Porras, 08”; label 3: “Voucher code M. Reemer / 319 / DNA voucher labcode MZH:Y0909”. Coll. QSBG.
Within Indascia, this exceptionally large species shares the presence of a posterior appendix on vein R4+5 only with Indascia spathulata sp. n. From that species, Indascia gigantica differs by tergite 2 being about 1.5 times as long as wide, and the basoflagellomere being 2 times as long as wide.
Adult male. Body size: 9.5 mm.
Head. Face occupying about 1/4 of head width in frontal view; black; entirely silvery white pilose. Gena black, white pilose. Oral margin not produced. Frons and vertex black; golden pilose, except for few black pile at ocellar triangle. Occiput black; yellowish pilose dorsally, white pilose ventrally. Eye bare. Antennal fossa about as high as wide. Antenna black; antennal ratio approximately as 4:1:4.
Thorax. Thorax black, except postalar callus and metanotum yellowish and posterior pleurites narrowly brownish along margins. Mesoscutum mixed golden and black pilose, with white pile at and around notopleuron. Postpronotum whitish pilose. Postalar callus black pilose anteriorly, yellow pilose posteriorly. Scutellum somewhat triangular, without calcars; black pilose dorsally, golden pilose along lateral and posterior margins. Anepisternum with deep sulcus separating anterior and posterior part; entirely long white pilose. Anepimeron entirely long white pilose. Katepisternum long white pilose dorsally, bare ventrally. Katatergum long microtrichose, anatergum short microtrichose. Calypter greyish. Halter pale yellow.
Wing: Hyaline, subtly darkened around apical crossveins and appendix of vein R4+5; microtrichose, except bare on cell sc, posterobasal 2/3 of cell c, basal 1/4 of cell r1, most of cell br except microtrichose along vena spuria, basal 5/6 of cell bm and basal 1/2 of cell cup.
Legs: Mid femur blackish, gradually turning yellow at apical 1/4; black pilose. Mid tibia yellow at basal 1/2, blackish at apical 1/2; black pilose. [Mid tarsus and other legs missing in holotype.] Coxae and trochanters black.
Abdomen. Tergites bronze-black. Tergite 1 long white pilose laterally, short black pilose sublaterally, bare medially. Tergite 2 with long white pile laterally on anterior 1/2, short black pilose over dorsal surface, short golden pilose narrowly along posterior margin. Tergite 3 long white pilose laterally on anterior 1/2, short black pilose over dorsal surface, long golden pilose on posterior 1/3. Tergite 4 with pilosity more or less as tergite 3, but much more sparse. Sternites blackish brown; sternite 1 bare; sternites 2-4 short black pilose anteriorly, long white pilose posteriorly. Male genitalia as in Fig. 112.
Female. Unknown.
The specific epithet (adjective) refers to the large size of this species in comparison with other known species of Indascia.
In the holotype, the only specimen available, the posterior appendix of vein R4+5 is composed of two short vein stumps, which are confluent at their apices, forming a triangle with part of vein R4+5. This is unusual, although similar aberrations can be found in single specimens of Microdontinae from different genera and species groups. Whether the venation as found in the holotype is representative of this species remains uncertain.
urn:lsid:zoobank.org:act:4E7D2BF5-2BD0-4D88-B5D5-D6FCD848DA8D
http://species-id.net/wiki/Indascia_spathulata
Figs 113–118HOLOTYPE. Adult male. VIETNAM. Label 1: “C. VIETNAM: Ha Tinh / Vu Quang N.P., 166 m, N 18° / 17'39"E 105°25'27", 24.ix. - / 5.x.2009, Mal. tr. 12, RMNH’09 / C. v. Achterberg & R. de Vries”; label 2: “Voucher code M. Reemer / 285 / DNA voucher labcode MZH:Y1100”. Coll. RMNH.
Within Indascia, this species only shares the presence of an appendix on vein R4+5 with Indascia gigantica sp. n. From that species, Indascia spathulata differs by tergite 2 being more than twice as long as wide, and the basoflagellomere being 5 times as long as wide.
Adult male. Body size: 6 mm.
Head. Face occupying slightly less than 1/3 of head width in frontal view; black; entirely silvery white pilose. Gena black, white pilose. Oral margin not produced. Frons and vertex black; yellowish white pilose, except for few black pile at ocellar triangle. Occiput black; black pilose dorsally, yellowish pilose laterally and ventrally. Eye bare. Antennal fossa about as high as wide. Antenna black; basoflagellomere with dorsal margin somewhat concave; antennal ratio approximately as 5:1:9.
Thorax. Thorax black, except postalar callus and postpronotum yellowish brown and ventral pleurae brown. Mesoscutum black pilose, except yellow pilose laterally. Postpronotum and postalar callus yellow pilose. Scutellum semicircular, without calcars; yellow pilose. Anepisternum weakly sulcate; dorsal 3/5 yellowish pilose, ventral 2/5 bare. Anepimeron entirely long yellowish white pilose. Katepisternum long white pilose dorsally, bare ventrally. Katatergum long microtrichose, anatergum short microtrichose. Calypter greyish. Halter yellowish white.
Wing: Hyaline; microtrichose, except bare on cell sc, basally on cell r1 along vein Rs, basal 1/2 of cell br, basal 3/4 of cell bm, anteriorly on cell cup along vein CuA.
Legs: pale yellow, except blackish brown on basal 1/3 of front, basal 3/4 of mid and most of hind femur (except extreme base and apex yellow in the latter), and distal 2/3 of hind tibia; yellow pilose, except black pilose on 4th and 5th tarsomere of front and mid leg, and on dark parts of hind femur and tibia. Coxae blackish brown; yellow pilose. Trochanters yellow; yellow pilose.
Abdomen. Tergites black, except anterior 1/4 of tergite 3 yellow and narrow anterior margin of tergite 4 yellow. Tergites 1 and 2 white pilose. Tergite 3 white pilose on yellow part, black pilose on black part. Tergite 4 black pilose, except white pilose in anterolateral corners and along posterior margin. Sternites black, except sternite 3 yellow on anterior 1/4. Sternite 1 bare. Sternite 2 white pilose. Sternite 3 white pilose on yellow part and along lateral margins. Sternite 4 black pilose, except white pilose along anterior margin. Male genitalia as in Fig. 118.
Female. Unknown.
Even more so than its congenerics, this species has a spoon-shaped abdomen, due to the strongly constricted second segment. This character inspired its name: spathulata (Latin adjective for ‘spatulate’, spoon-shaped).
urn:lsid:zoobank.org:act:8F15F979-79AC-4F43-815A-1547719D0808
http://species-id.net/wiki/Kryptopyga_sulawesiana
Figs 126–129, 131HOLOTYPE. – Male. Label 1: “INDONESIA; N. Sulaw.; / 20 km N. Bitung: Tang- / koko N.P.; 0–200 m; / 1°N, 125°12 E; 19 / IV 1988; R. Hensen.” Coll. RMNH.
This species differs from Kryptopyga pendulosa by the less modified abdomen: tergite 4 is not perpendicular to tergite 3 and sternite 4 is well visible in ventral view.
Adult male. Body size: 14 mm.
Head. Face occupying about 2/5 of head width in frontal view; black on median 1/2, pale brown on lateral 1/4; entirely long appressed yellowish pilose, golden on ventral half. Gena widely developed; blackish; long yellow pilose. Oral margin anteriorly notched, laterally produced. Frons black; short golden pilose. Vertex strongly swollen; black; short golden pilose anteriorly, long black pilose posterior to ocellar triangle. Ocellar triangle not elevated. Occiput strongly swollen dorsally, narrow laterally; black; black pilose dorsally, golden pilose ventrally. Eye bare. Antennal fossa about as high as wide. Antenna blackish brown, scape a little paler basally; ratio of lengths of scape and basoflagellomere approximately as 1:4, pedicel very short; basoflagellomere very long (4 mm), parallel-sided, with very long black pilosity, about 1, 5 times as long as width of basoflagellomere. Arista absent.
Thorax. Mesoscutum black; black pilose, except for some golden pile along transverse suture and along lateral margins. Postpronotum and postalar callus brown, black pilose. Scutellum yellow; black pilose. Pleuron blackish brown. Anepisternum with deep sulcus separating posterior from anterior part; mixed yellow and black pilose anteriorly, black pilose posterodorsally, yellow pilose posteriorly. Anepimeron entirely long yellow pilose. Katepisternum long pale yellow pilose dorsally and ventrally. Katatergum long microtrichose, anatergum short microtrichose. Calypter greyish yellow. Halter yellow.
Wing: hyaline, slightly darkened anteroapically; microtrichose, except bare on 1st and cell c, basal 1/3 of cell r1, basal 1/4 of cell r2, basal 1/2 of cell r4+5, basal 1/2 of cell dm, entirely on cells R and BM, entirely on cell br and BM, anterobasally on cell dm, most of cell cup and most of alula (only microtrichose along margins).
Legs: Brown, more blackish on femora and fore- and mid-tibiae; femora pale pilose anteriorly, black pilose posteriorly; tibiae and tarsi yellow pilose.
Abdomen. Elongate, more or less oval, with widest point at posterior margin of tergite 2; high in lateral view; tergites 3 and 4 not fused, with posterior margin of tergite 3 strongly overlapping tergite 4. Tergites blackish, except terigte 1 yellowish brown and other tergites narrowly yellowish brown along margins; short black pilose, except longer yellowish pilose along lateral margins of all tergites and posterolateral margins of tergites 3-4. Sternite 1 blackish; bare. Sternites 2-4 dark brown on anterior 2/3, yellow on posterior 1/3; entirely long yellow pilose. Genitalia as in Fig. 131.
Female. Unknown.
The specific epithet (noun in the genitive case) is derived from the Indonesian island Sulawesi, the type locality.
urn:lsid:zoobank.org:act:596FD7FE-BBBE-4990-B2C9-4658FFBF0C05
http://species-id.net/wiki/Masarygus_palmipalpus
Figs 140–146HOLOTYPE. Adult male. PERU. Label 1: “PERU. Madre de Dios, Rio / Tambopata, Sachavacayoc Centre / 12°51'S–69°22'W. Mal. trap / 28–30.X.2008. Leg. J.T. Smit”. Coll. RMNH (preliminary deposition, to be transferred to relevant Peruvian collection later).
This is the only known species of Microdontinae in which the antenna of the male is furcate into five branches.
Adult male. Body size: 4 mm.
Head. Head unusually flat. Face wide: occupying about 3/4 of head width in frontal view; somewhat concave laterally; yellow; yellow pilose, except black pilose laterally on dorsal 1/2. Gena yellow; yellow pilose. Oral margin not produced; oral opening barely visible; mouth parts undeveloped. Frons brown; black pilose; very short; distance between frontal ocellus and antennal fossa shorter than height of antennal fossa. Vertex blackish brown medially, yellow laterally; black pilose; ocelli arranged almost in a straight line, with frontal ocellus weakly developed, much smaller than the other two. Occiput yellow; black pilose dorsally, yellow pilose ventrally. Eye bare. Antennal fossa about 1, 5 times as wide as high. Antenna black; black pilose; ratio of scape:basoflagellomere approximately as 1:8; pedicel very short. Basoflagellomere furcate into five branches, four of which about equally long, the fifth branches off from one of the other at about ¼ from the base of the segment, with a length of about 2/5 of the other branches. Arista absent.
Thorax. Mesoscutum black, except narrowly pale yellow along margins; black pilose. Postpronotum pale yellow; bare. Postalar callus pale yellow; black pilose. Scutellum black; black pilose; semicircular; without calcars; flat, appearing even slightly concave; smooth and shining along margins, dull dorsally due to micropunctation; black pilose. Anepisternum pale yellow along dorsal margin, brown otherwise; with sparse long black pile, also ventrally; without sulcus. Other pleurae yellowish to brown; bare (also without microtrichia). Calypter pale yellow. Halter pale yellow with greyish margin.
Wing: Hyaline; microtrichose, except bare on cell sc and basal 1/4 of cell cup.
Legs: Front and mid leg pale yellow, except dark brown on basal 3/4; black pilose. Hind leg dark brown, except fifth tarsomere yellow; black pilose. Front coxa exceptionally long: about 4/5 of length of femur, longer than tibia; pale brown; bare. Other coxae and trochanters shorter; pale brown; very sparsely black pilose.
Abdomen. Strongly flattened dorsoventrally. Tergite 1 blackish; black pilose; medially interrupted by the whitish antetergite, which is almost entirely fused with the tergite. Tergites 2 and 3 whitish, except black on lateral 1/5, the black part most narrow at posterior margin; black pilose. Tergite 4 black, except for a pair of whitish, submedian, oval maculae at posterior 1/2. Sternite 1 whitish; bare. Sternite 2 whitish; yellow pilose. Sternite 3 whitish, except for lateral dark brown, round macula at anterior 1/2, of about 1/4 of tergite width; yellow pilose, except black pilose anteromedially. Sternite 4 whitish, except for pair of dark brown, oval maculae, almost confluent medially; black pilose anteriorly, yellow pilose posteriorly. Male genitalia as in Fig. 146.
Female. Unknown.
The specific epithet (noun in apposition) is composed of the Latin words palma (hand) and palpus (feeler, here interpreted as antenna). The name refers to the hand-like antenna of the male of this species.
urn:lsid:zoobank.org:act:5282F07F-EAD6-4A3D-92BD-18A5C32AA2E0
http://species-id.net/wiki/Mermerizon_inbio
Figs 157–162HOLOTYPE. COSTA RICA. Male. Label 1: “COSTA RICA. Prov. Guanacaste, P.N. / Rincón de la Vieja, Send. a las aguas / termales, 900–1000 m, 6–7 OCT / 2001. D. Briceño, Red con Aguamiel. / L_N_305843_392970 #64950”; label 2: “INB0003380896 / INBIOCRI COSTA RICA”; label 3 (red): “Ultimo especimen en / BD A. Lépiz / 2-7-2002” / other side: “?MCR-25”. Coll. INBIO.
Distinguished from the other two known (yet undescribed) species of Mermerizon by the black pilose mesoscutum.
Adult female. Body size: 7, 5 mm.
Head. Face occupying about 1/4 of head width in frontal view; yellow; yellow pilose, with narrow bare median line on dorsal half. Gena yellow. Frons black; yellow pilose laterally, black pilose posteriorly. Vertex dark yellow, except black at and around ocellar triangle; black pilose. Occiput black, except yellow posteriad of vertex; black pilose on dorsal half, yellow pilose on ventral half. Eye bare. Antennal fossa about as high as wide. Antenna with scape dark brown, pedicel and basoflagellomere yellowish brown; antennal ratio approximately as 4:1:4.
Thorax. Scutum blackish brown, except yellow on notopleuron and around postpronotum and postalar callus; black pilose. Postpronotum, postalar callus and scutellum yellow; black pilose. Scutellum semicircular, without calcars, Anepisternum blackish brown; convex, without sulcus; black pilose on anterior part and along posterior margin, widely bare in between. Anepimeron brown; black pilose on dorsal 1/4. Katepisternum yellow dorsally, brown ventrally; bare. Katepimeron yellow. Katatergum long microtrichose. Anatergum short pale microtrichose. Calypter blackish. Halter yellowish brown.
Wing: hyaline; microtrichose, except bare on cell bc, basal 1/4 of cell br, basal 1/3 of cell bm, anterobasal 1/4 of cell cup.
Legs: Front and mid legs yellowish brown; black pilose. Hind leg blackish brown, except basal 1/2 of tibia and apical four tarsomeres yellowish brown. Front and mid coxae and trochanters yellowish brown; yellow pilose apically. Hind coxa and trochanter dark brown; black pilose.
Abdomen. Tergites and sternites yellowish; yellow pilose, except sternite 1 bare. Genitalia as in Fig. 162.
Female. Unknown.
The specific epithet (noun in apposition) is based on InBio, an acronym of Instituto Nacional de Biodiversidad, the Costa Rican institute which holds the holotype of this species.
urn:lsid:zoobank.org:act:5468A969-1FE2-4D32-969E-B878838546C0
http://species-id.net/wiki/Metadon_achterbergi
Figs 163–166HOLOTYPE. Adult female. VIETNAM. Label 1: “C. VIETNAM: Ha Tinh / Vu Quang N.P., 53 m, N 18° / 20'50" E105°26'37.8", 22.ix.- / 6.x.2009, Mal. trap 1, RMNH-09 / C. v. Achterberg & R. de Vries”. Coll. RMNH. Voucher code M. Reemer: 284. DNA voucher labcode MZH:Y1086.
Within Metadon, five other described Oriental species have a dark (sub)apical wingspot. These species are listed here (in parentheses a character is given that distinguishes them from Metadon achterbergi sp. n.): Microdon auricinctus Brunetti, 1908 (tergite 4 red); Metadon bicoloratus Hull, 1944 (thorax and abdomen without fasciae of golden pile); Metadon fuscicornis Sasakawa, 1960 (wing infuscated at entire apical half); Metadon pendleburyi Curran, 1931 (thorax and abdomen without fasciae of golden pile); Metadon wulpii Mik, 1899 (mesoscutum without fascia of golden pile along transverse suture, scutellum reddish brown).
Adult female. Body size: 13 mm.
Head. Face occupying about 1/3 of head width in frontal view; dark brown; golden pilose, very narrowly bare medially on ventral half. Gena brown, golden pilose. Lateral oral margin produced. Frons, vertex and occiput brown; golden pilose, except occiput ventrally white pilose. Eye bare. Antennal fossa about as high as wide. Antenna black; antennal ratio approximately as 4:1:3.
Thorax. Mesoscutum blackish brown; golden pilose, with transverse fasciae of thicker golden pile along anterior margin, transverse suture (fascia medially interrupted) and posterior margin. Postpronotum and postalar callus yellow; golden pilose. Scutellum semicircular; blackish brown; golden pilose. Anepisternum with deep sulcus; yellowish brown; entirely golden pilose. Anepimeron yellowish brown; entirely pilose. Katepisternum blackish brown; golden pilose dorsally, very sparsely pilose ventrally. Other pleurites yellowish brown. Katatergum long microtrichose, anatergum short microtrichose. Calypter and halter yellowish.
Wing: Yellow on basal 2/3, blackish on apical 1/3, with colouration in posterior half less conspicuous. Microtrichose, except bare on cell bc, narrowly along vein Rs in cell r1, basal 3/4 of cell br, basomedian 3/4 of cell bm, anterobasal 1/4 of cell cup, and basomedian 9/10 of alula.
Legs: Yellow; yellow pilose. Front coxa yellow, mid and hind coxae blackish brown; yellowish pilose. Trochanters blackish brown; yellowish pilose.
Abdomen. Tergites black. Tergites 1 and 2 golden pilose. Tergite 3 golden pilose anterolaterally; black pilose otherwise. Tergite 4 with fascia of golden pile along anterior margin, laterally widening and expanding along lateral margin, and with pair of sublateral oblong maculae of golden pile, black pilose in between. Tergite 5 golden pilose anterolaterally, black pilose otherwise. Sternites blackish brown; pale pilose, except sternite 5 mostly black pilose.
Male. Unknown.
This species is named after its collector, Dr. C. van Achterberg, in acknowledgment of the many ways in which he has been helpful to the senior author during his PhD work. The epithet is a noun in the genitive case.
urn:lsid:zoobank.org:act:2B1D3B15-0332-49CA-8B12-F6ED443CDC38
http://species-id.net/wiki/Microdon_hauseri
Figs 196–199, 212HOLOTYPE. Adult male. CHINA. Label 1: “Yunnan. Tengchong / 50 km NNW: Houqiao / N25.388° E 98.211° / 1700 m / 01.VI.2009 leg. / Blank, Liston, Taeger / 008 China”; label 2: “Voucher code M. Reemer / 302 / DNA voucher labcode MZH:Y1096”. Coll. CSCS.
In the keys of Shiraki (1968), Huo et al. (2007) and – depending on how characters are interpreted – Hironaga and Maruyama (2004), this species keys to Microdon auricomus Coquillet, 1898, from which it differs by the largely orange legs and the long, orange-golden pilosity on the anterodorsal part of the hind femur. These characters also apply for distinguishing Microdon hauseri sp. n. from Microdon murayamai Hironaga & Maruyama, 2004, to which specimens of the species will key in the key of Hironaga and Maruyama (2004). The same characters apply for separating it from Microdon lateus Violovitsh, 1975, to which it keys using Violovitsh (1983). In the key of Shiraki (1930) this species keys to Microdon formosanus Shiraki, 1930, from which it differs by the black pilosity medially on the mesoscutum (entirely pale in Microdon formosanus).
Adult male. Body size: 12.5 mm.
Head. Face occupying about 1/3 of head width in frontal view; black; entirely yellowish pilose. Gena black, yellowish pilose. Oral margin not produced. Frons black; black pilose, except narrowly yellow pilose along lateral and posterior margins. Vertex black; black pilose, except narrowly yellow pilose along all margins. Occiput black; yellow pilose. Eye bare. Antennal fossa about as high as wide. Antenna black; antennal ratio approximately as 3.5:1:2.5.
Thorax. Entire thorax blackish with bronze hues. Mesoscutum black pilose medially, widely yellow pilose along margins. Postpronotum, postalar callus and scutellum yellow pilose. Scutellum trapezoid with slightly concave posterior margin; without calcars. Anepisternum yellow pilose anteriorly, mixed black and yellow pilose posteriorly, with widely bare part in between; with shallow sulcus separating anterior from posterior part. Anepimeron entirely yellow pilose. Katepisternum yellow pilose dorsally, very sparsely yellow pilose ventrally. Katatergum long microtrichose, anatergum short microtrichose. Calypter and halter pale yellow.
Wing: Hyaline, subtly darkened around apical crossveins; microtrichose, except bare on basal 3/5 of cell br and basomedian 1/3 of alula.
Legs: Orange, except basal 1/4 of femora blackish, apex of femora narrowly darkened and tibiae dorsally darkened. Front femur black pilose, except for patch of orange-golden pile anterobasally; mid femur orange-golden pilose anteriorly and posteriorly on basal 2/3, with patch of orange-golden pile anteroventrally on basal 1/4, black pilose dorsally and ventrally; hind femur with long orange-golden pile anterodorsally and posteriorly, with orange-golden pile on basal 1/3, black pilose otherwise. Front and mid tibia orange-golden pilose, except black pilose dorsally. Hind tibia orange-golden pilose (long dorsally, short ventrally), except black pilose laterally. Tarsi black pilose. Coxae and trochanters black; pale pilose.
Abdomen. Tergites black with bronze hues. Tergites 1 and 2 golden pilose. Tergite 3 golden pilose on lateral 1/4, orange-golden pilose medially (colour transition gradual). Tergite 4 orange-golden pilose, except for pair of submedian patches of black pile on anterior 1/2; each about as wide as 1/4 of the tergite. Sternites black with bronze hues; entirely whitish to golden pilose. Male genitalia as in Fig. 212.
Female. Unknown.
This species is named after Martin Hauser, in acknowledgement for the many interesting specimens of Microdontinae he sent to the author. The epithet is a noun in the genitive case.
urn:lsid:zoobank.org:act:B34669D3-D2D9-4CE8-8B96-CBF2A61D1DA3
http://species-id.net/wiki/Microdon_mandarinus
Figs 200–205, 213HOLOTYPE. Adult male. CHINA. Label 1: “Yunnan: Deqin / 10 km SW: Meili mts. / N28.423°, E98.868° / 2700 m / 20.VI.2009 leg. / Blank, Liston, Taeger / 048 China”; label 2: “Voucher code M. Reemer / 299 / DNA voucher labcode MZH:Y1093”. Coll. CSCS.
PARATYPE. CHINA. Label 1: “Yunnan: / Deqin 33 km SE / N28.282°, E99.162° / 3200 m / 18.VI.2009 leg. / Blank, Liston, Taeger / 040 China”. Coll. CSCS.
The orange colouration of large parts of this species’ body, most notably its head, legs and the lateral parts of the tergites, precludes confusion with any other known Palaearctic or Oriental species of Microdon s.s.
Adult male. Body size: 11 mm.
Head. Face occupying about 1/3 of head width in frontal view; orange yellow; entirely yellow pilose. Gena black, yellow pilose. Oral margin laterally weakly produced. Frons black; yellow pilose. Vertex yellow; yellow pilose. Occiput black; yellow pilose. Eye almost bare, sparse and short pile visible only under high magnification. Antennal fossa about as high as wide. Antenna pale brown; antennal ratio approximately as 3:1:2.5.
Thorax. Mesoscutum blackish bronze with green metallic hues, except yellow along lateral margins; entirely yellow pilose. Postpronotum, postalar callus and scutellum yellow; yellow pilose. Scutellum trapezoid with slightly concave posterior margin; with minute, barely discernable posterolateral calcars, their mutual distance about equal to 1/3 of width of scutellum. Pleuron blackish, except anepisternum anterodorsally with small yellow spot and katatergum medially with small yellow spot; all pilosity yellow. Anepisternum with deep sulcus separating anterior from posterior part; pilose anteriorly and posteriorly, with widely bare part in between. Anepimeron entirely pilose. Katepisternum pilose dorsally; bare ventrally. Katatergum long microtrichose, anatergum short microtrichose. Calypter and halter yellowish white.
Wing: Hyaline; microtrichose, except bare on posterobasal 1/4 of cell br.
Legs: Yellow, except narrowly blackish around basal cicatrix on femora; yellow pilose. Coxae and trochanters yellow, except hind coxa black on basal half; yellow pilose.
Abdomen. Tergite 1 black; yellow pilose. Tergite 2 black, except orange yellow on lateral 1/6; erect yellow pilose , except for fascia of appressed golden pile along posterior margin. Tergite 3 medially with semicircular black mark, anteriorly as wide as the black part on tergite 2, posteriorly narrow and just reaching posterior margin; laterally orange yellow; short black pilose on most of anterior half, except yellow pilose along lateral margins, with fascia of appressed golden pile along posterior margin. Tergite 4 largely orange yellow, except for vaguely defined blackish mark anteromedially; largely short yellow pilose, except for anterolateral patches of black pile. Sternite 1 black; yellow pilose. Sternite 2 and 3 yellow, except blackish near lateral margins; yellow pilose. Sternite 4 yellow; yellow pilose. Male genitalia as in Fig. 213.
Female. As male, except for the following differences. Body size: 14 mm. Frons largely yellow, except for small triangular black area posteriad of lunula. Antenna: scape and pedicel yellowish. Mesoscutum with pair of small submedian yellow spots at posterior margin. Scutellum without any sign of calcars. Anepimeron, dorsal part of katepisternum, katepimeron, katatergum and anatergum yellow. Tergite 4 with fascia of appressed golden pile on posterior half. Tergite 5 largely orange yellow, except blackish anteromedially; entirely appressed golden pilose.
The species name (adjective) refers to ‘mandarin’, which has a number of meanings. It’s an orange citrus fruit, it’s the most spoken language in China, and it used to be a high governmental function in imperial China. The name is considered appropriate for this species because of the characteristic orange colour of several body parts and the Chinese origin of the type material.
urn:lsid:zoobank.org:act:9A81751D-97B5-445A-9F78-9A5E5067E394
http://species-id.net/wiki/Microdon_yunnanensis
Figs 207–210, 214HOLOTYPE. Adult male. CHINA. Label 1: “Yunnan: Tengchong / 25 km NNW / N25.189°, E98.333° / 1900 m. / 01.VI.2009 leg. / Blank, Liston, Taeger / China 010”; label 2: “ Voucher code M. Reemer / 301 / DNA voucher labcode MZH:Y1095”. Coll. CSCS.
This species keys to Microdon japonicus Yano, 1915 in the keys of Huo et al. (2007) and Shiraki (1930, 1968). From that species it is distinguished by the entirely yellow pilose mesoscutum (with patches of black pile in Microdon japonicus). In the key of Hironaga and Maruyama (2004) it keys to Microdon kidai Hironaga & Maruyama, 2004, from which it differs by its partly yellow legs (entirely black in M. kidai). In the key of Violovitsh (1983) this species keys to Microdon eggeri Mik, 1897 (= Microdon analis (Macquart, 1842)), from which it differs by its pale brown scutellum (black in Microdon analis) and the shape of tergite 2, which is at its widest clearly before the posterior margin (widest at posterior margin in Microdon analis).
Adult male. Body size: 11 mm.
Head. Face occupying a little less than 1/2 of head width in frontal view; black; entirely golden yellow pilose. Gena black, golden yellow pilose. Oral margin not produced. Frons, vertex and occiput black; golden yellow pilose. Eye bare. Antennal fossa about as high as wide. Antenna black; antennal ratio approximately as 2.5:1:1.5.
Thorax. Entire thorax blackish with bronze hues, except scutellum brownish; all pilosity yellow. Scutellum trapezoid with slightly concave posterior margin; with slender calcars as long as 1/5 of length of scutellum, their mutual distance about equal to 1/3 of width of scutellum. Anepisternum with shallow sulcus separating anterior from posterior part; pilose anteriorly and posteriorly, with widely bare part in between. Anepimeron entirely pilose. Katepisternum pilose dorsally and ventrally. Katatergum long microtrichose, anatergum short microtrichose. Calypter and halter yellowish white.
Wing: Hyaline, subtly darkened around apical crossveins; microtrichose, except bare on basal 1/2 of cell br.
Legs: Black, except basal 3/5 of tibiae and ventral side of tarsi yellow; yellow pilose. Coxae and trochanters black; yellow pilose.
Abdomen. Tergites black. Tergites 1 and 2 yellow pilose. Tergite 3 black pilose, except narrowly whitish pilose along lateral and posterior margins. Tergite 4 black pilose, except narrowly whitish pilose along lateral margins and whitish pilose on posterior 1/3. Sternites black; whitish pilose. Male genitalia as in Fig. 214.
Female. Unknown.
This species is named after the Chinese province of Yunnan, in which it was found. The epithet is a noun in the genitive case.
urn:lsid:zoobank.org:act:C4B02C42-9193-4281-AB2D-13194B38A444
http://species-id.net/wiki/Paramixogaster_piptotus
Figs 272–275HOLOTYPE. Female. Label 1: “Madagascar / Bekily / Reg sud de l’ile”; label 2 (blue): “Museum Paris / I.37 / A. Seyrig”; label 3: “Ceratrichomyia / behara type du genre [female sign] Séguy 1950”. Coll. MNHN. [this specimen is one of the paralectotypes of Ceratrichomyia behara; see redescription of that species above]
This is the only known species of Paramixogaster with a pair of submedian vittae of golden pilosity on the posterior half of the mesoscutum.
Adult female. Body size: 7 mm.
Head. Face occupying about 3/5 of head width in frontal view; yellow; entirely yellow pilose. Gena yellow. Lateral oral margins very slightly produced. Frons and vertex yellow; yellow pilose. Occiput yellow; dorsally wide and yellow pilose, ventrally narrow and whitish pilose. Eye bare. Antennal fossa about as wide as high. Antenna orange; antennal ratio approximately as 1:0, 25:6. Basflagellomere elongate; with sensory pit at apical 1/7. Arista yellow, about 2/5 of length of basoflagellomere.
Thorax. Postpronotum yellow; bare. Mesoscutum reddish brown; short yellow pilose, with lateral fasciae of dense golden pile along transverse suture and with two vittae of dense golden pile on posterior half. Postalar callus and scutellum reddish brown; short yellow pilose. Scutellum without calcars. Pleuron reddish brown. Anepisternum with deep sulcus separating anterior and posterior part; white pilose, except with golden pilosity along posterior margin, as an extension of the golden fascia from the mesonotal transverse suture. Anepimeron entirely white pilose. Katepisternum white pilose dorsally; bare ventrally. Katatergum long microtrichose, anatergum short microtrichose. Other pleurites bare. Calypter and halter yellow.
Wing: hyaline; microtrichose, except bare on cell bc, basal 1/2 of cell c, basally on cell r1 along vein Rs, almost entirely on on cells R, BM andl CuP, on alula except along margins.
Legs: Yellow, except: front femur brownish on basal half, middle and hind femur dark brown on basal 4/5. Legs entirely pale pilose. Coxae and trochanters brown; pale pilose.
Abdomen. Constricted at 2nd segment, narrowest at anterior margin of tergite 2, widest at tergite 3 (slightly wider than thorax). Ratio of tergal lengths approximately as 1:4:3:3:3. Tergite 1 dark brown; pale pilose. Tergite 2 dorsoventrally flattened, dark brown with large, oblique yellow maculae over entire length, which are interconnected anteriorly, leaving anterolateral corners and a large posteriomedian triangle dark brown; yellow pilose. Tergite 3 and 4 dark brown and short yellow pilose; with fasciae of golden pile along posterior margins; tergite 4 also with posterolateral margins yellow in ground colour. Tergite 5 brown with posterior 2/5 and median part yellow; yellow pilose. Sternite 1 dark brown; bare. Sternite 2 yellow; yellow pilose. Other sternites brown; yellow pilose.
Male. Unknown.
The specific epithet (adjective) is derived from the Greek word piptotos (that which has fallen). This name refers to the fact that this species ‘fell’ out of the genus Ceratrichomyia, for the holotype is also part of the paratype series of Ceratrichomyia behara Séguy, 1951.
This description is based on the female paratype of Ceratrichomyia behara. For discussion see genus account of Ceratrichomyia.
urn:lsid:zoobank.org:act:F75F1363-5403-4D38-A7C6-97B79488059A
http://species-id.net/wiki/Piruwa_phaecada
Figs 299–306HOLOTYPE. Adult male. PERU. Label 1: “PERU. Madre de Dios, Rio / Tambopata, Sachavacayoc Centre / 12°51'S, 69°22'W. Mal. trap / 4-10.IX.2009. Leg. J.T. Smit”; label 2: “Voucher code M. Reemer / 287”. Coll. RMNH (preliminary deposition, to be transferred to relevant Peruvian collection later).
PARATYPES. 2 adult females with label data as holotype, but collection dates are 28–30.X.2008 and VIII.2009. Coll. RMNH & J.T. Smit.
See generic key and genus account of Piruwa (only one species known).
Adult male. Body size: 4 mm.
Head. Face occupying about 1/6 of head width in frontal view; black; entirely white pilose. Gena hardly developed; black, white pilose. Oral margin not produced. Frons, vertex and occiput black; white pilose. Eye bare. Antennal fossa about as high as wide. Antenna: scape black, pedicel and basoflagellomere pale brown; antennal ratio approximately as 2.5:1:3.
Thorax. Black. Mesoscutum short appressed yellowish pilose, except for sparse bristly black pile anterolaterally. Postpronotum bare. Postalar callus black pilose dorsally, pale pilose laterally. Scutellum semicircular; without calcars; black; short pale pilose and with a few long, bristly, pale setae along posterior margin. Anepisternum convex, without sulcus; pale pilose on dorsal half. Katatergum and anatergum short pilose. All other pleurites bare. Calypter grey. Halter yellow with blackish knob.
Wing: Hyaline. Microtrichose, except bare cell bc, basal 1/2 of cell br and cell bm, basal 1/3 of cell cup.
Legs: Tibiae and femora black. Tarsi whitish yellow, except first tarsomere of hind leg black. Legs black pilose, except tarsi of front and mid leg dorsally yellow pilose. Coxae and trochanters blackish brown. Coxae white pilose apically. Trochanters bare.
Abdomen. Tergites black. Tergites yellowish pilose, except tergite 4 laterally and posteriorly mixed black and yellow pilose. Sternites blackish brown; sternite 1-2 bare; sternites 3-4 short black pilose. Genitalia as in Fig. 304.
Female. As male, except for following differences (based on paratype collected VIII.2009). Face golden yellow pilose. Mesoscutum and scutellum mixed golden yellow and black pilose. Pleuron partly brownish. Anepisternum black pilose dorsally. Anepimeron with bristly black pile along dorsal margin. Coxae apically black pilose. Sternite 5 blackish; short black pilose, withlong, bristly black pile along posterior margin. The other female paratype is apparently a teneral specimen, as parts of its body are yellowish brown.
The specific epithet phaecada (adjective) is derived from the Greek word phaikas, which is a kind of white shoe. The name refers to the whitish yellow tarsi of the species that contrast with the entirely black rest of the body.
urn:lsid:zoobank.org:act:BFB07B79-EE6E-4391-AB42-AB3F970AEA5B
http://species-id.net/wiki/Pseudomicrodon_polistoides
Figs 307–311HOLOTYPE. Adult female. PERU. Label 1: “PERU. Madre de Dios, Tambopata / Sachavacayoc Centre, Quebrada / trail. S12°51'20.1"–W69°22'20.1". / Alt. 166 m. Malaise trap / 14–25.VI.2010. Leg. J.T. Smit”. Label 2: “Voucher code M. Reemer / 346 / DNA voucher labcode MZH:Y1319”. Coll. RMNH (preliminary deposition, to be transferred to relevant Peruvian collection later).
In three other described Pseudomicrodon speciesthe alula is completely microtrichose: Pseudomicrodon chrysostypus (Thompson, 2004), Pseudomicrodon pilosops (Marinoni, 2004) and Pseudomicrodon smiti sp. n. From these species, Pseudomicrodon polistoides sp. n. differs by the entirely orange coloured abdomen, as well as by the yellow median vitta on the mesoscutum.
Adult female. Body size: 12.5 mm.
Head. Face occupying approximately 1/3 of head width in frontal view; yellow; yellow pilose on lateral 1/3, black pilose on median 1/3. Gena yellow; yellow pilose. Lateral oral margins weakly produced. Frons yellow, except for black markings directly laterad of antennal fossa; yellow pilose, except for sparse black pile at black markings. Vertex yellow, except for black markings at and around ocellar triangle and posterolaterally; bare on anterior 1/3, black pilose on posterior 2/3. Occiput yellow, except black adjacent to black markings on vertex; yellow pilose, except black pilose directly posteriad of vertex. Eye almost bare, with very sparse and short white pile. Antennal fossa about as wide as high. Antenna orange yellow, scape a little darker; antennal ratio approximately as 5:1:6; longer than distance between antennal fossa and anterior oral margin.
Thorax. Mesoscutum black with widely yellow margins and wide median yellow vitta over entire length, also narrowly yellow along transverse suture. Black pilose, except for fasciae of orange golden pile along anterior margin, transverse suture and posterior margin, as well as along posterolateral margin. Postpronotum and postalar callus yellow; black pilose. Scutellum semicircular; yellow; black pilose, except sparsely golden pilose anterolaterally; without calcars. Pleuron yellow, except dorsomedial and posterior parts of anepisternum partly blackish, and anatergum and lateral margins of mediotergite blackish. Anepisternum sulcate; mixed orange and black pilose anterodorsally, black pilose posteriorly, widely bare in between. Anepimeron entirely yellow pilose. Katepisternum yellow pilose dorsally, bare ventrally. Katatergum long microtrichose, anatergum short microtrichose. Calypter and halter yellowish white.
Wing: Yellowish to brown in cells bc and c, cell br, R1, R2+3, anteriorly in cell r4+5, hyaline in other parts. Microtrichose, except bare on cell bc, posterobasal 1/4 of cell br, posterobasal 1/4 of cell bm and basal 1/10 of cell cup.
Legs: Yellow; yellow pilose, except femora posteriorly black pilose and hind tarsus dorsally black pilose. Front and mid coxae and trochanters yellow; yellow pilose. Hind coxa yellow anteriorly, blackish brown laterally and posteriorly; yellow pilose, except black pilose apically and laterally. Hind trochanter brownish, mixed yellow and black pilose.
Abdomen. Constricted, about as wide as thorax, with narrowest point in posterior 3/4 of tergite 2. Tergites orange, except tergite 1 laterally and tergite 2 anterolaterally dark brown and tergites 2 and 3 with posterior margins yellow. Tergite 1 black pilose laterally, yellow pilose medially. Tergites 2-4 black pilose, except yellow pilose posteriorly. Tergite 5 yellow pilose, except black pilose medially. Sternite 1 brownish; bare. Other sternites yellow; yellow pilose.
Male. Unknown.
The specific epithet (adjective) emphasizes the resemblance of this species to certain Polistinae (Hymenoptera: Vespidae).
urn:lsid:zoobank.org:act:D6CF66F8-DD63-4075-8459-977E3181CB17
http://species-id.net/wiki/Pseudomicrodon_smiti
Figs 312–316, 320HOLOTYPE. Adult male. PERU. Label 1: “PERU. Madre de Dios, Tambopata / Sachavacayoc Centre, Bridge / Condonado trail, S 12°51'25.7” - / W 69°22'23.1”. Alt. 184 m. / 5.VI.2010. Leg. J.T. Smit”. Label 2: “Voucher code M. Reemer / 345 / DNA voucher labcode MZH:Y1318”. Coll. RMNH (preliminary deposition, to be transferred to relevant Peruvian collection later).
PARATYPES. A male and a female from same locality as holotype, collected on 10.VI and 8.VI.2010, respectively. Male in coll. J.T. Smit, female in coll. RMNH.
In three other described Pseudomicrodon speciesthe alula is completely microtrichose: Pseudomicrodon chrysostypus (Thompson, 2004), Pseudomicrodon pilosops (Marinoni, 2004) and Pseudomicrodon polistoides sp. n. From these species, Pseudomicrodon smiti sp. n. differs by the combination of the black postpronutum and the partly black hind tibia.
Adult male. Body size: 9.5 mm.
Head. Face occupying a little more than 1/4 of head width in frontal view; black, except yellow on lateral 1/5 in dorsal 2/3; entirely yellow pilose; medially with vitta of transversely wrinkled texture. Gena black; yellow pilose. Lateral oral margins weakly produced. Frons black; white pilose. Vertex black; bare on anterior half, black pilose on posterior half. Occiput black; golden pilose on dorsal half, silvery white pilose on ventral half. Eye almost bare, with very sparse and short white pile. Antennal fossa about 1.5 times as wide as high. Antenna black; antennal ratio approximately as 2.5:1:3.5; longer than distance between antennal fossa and anterior oral margin.
Thorax. Blackish brown with bronze and green metallic hues. Mesoscutum appressed black pilose, except for medially interrupted fasciae of appressed golden pile along anterior margin and transverse suture. Postpronotum and postalar callus black pilose. Scutellum semicircular; black pilose, except golden pilose posterolaterally; with calcars about as long as 1/3 of length of scutellum, with mutual distance approximately 1/4 of scutellar width. Anepisternum sulcate; black pilose anterodorsally, white pilose posteriorly, widely bare in between. Anepimeron entirely silvery white pilose. Katepisternum silvery white pilose dorsally, bare ventrally. Katatergum long microtrichose, anatergum short microtrichose. Calypter and halter yellowish white.
Wing: Hyaline, brownish in cells bc, c and sc, and cell r1. Microtrichose, except bare cell bc, posterobasal 3/4 of cell br, basal 2/3 of cell bm and basal 1/6 of cell cup.
Legs: Blackish brown, except whitish on basal 1/3 of mid tibia and basal 2/5 of hind tibia, paler brown on apical half of femora. Femora black pilose, except hind femur white pilose posteriorly and anterobasally. Tibiae white pilose, except black pilose ventrally, and mid tibia black pilose on apical 2/5. Tarsi dorsally black pilose. Coxae brown; front and mid coxae black pilose, hind coxa white pilose. Trochanters brown; black pilose.
Abdomen. Constricted, narrower than thorax, with narrowest point halfway of tergite 2. Tergites black, except tergite 2 largely occupied by pair of rectangular yellow maculae on basal 3/4. Tergite 1 long yellowish white pilose laterally, black pilose dorsally. Tergite 2 black pilose, except narrowly golden pilose along posterior margin. Tergite 3 black pilose, except white pilose along lateral margin and with medially interrupted fascia of golden pile along posterior margin. Tergite 4 black pilose, except white pilose anterolaterally and along lateral margin, and with pair of submedian vittae of golden pile on posterior 3/4, widening towards apex. Sternite 1 black; bare. Sternite 2 whitish yellow; bare. Sternites 3 and 4 black; black pilose. Male genitalia as in Fig. 320.
Female. Body size: 11 mm. As male, except for usual sexual differences. Tergite 5 golden pilose medially, white pilose laterally.
This species is named after John T. Smit, who collected this species in Peru, along with several other interesting Microdontinae. The epithet is a noun in the genitive case.
urn:lsid:zoobank.org:act:E823BA6D-86C3-4014-950D-AEBE9A00B943
http://species-id.net/wiki/Rhopalosyrphus_ecuadoriensis
Figs 332–336, 352HOLOTYPE. Adult male. ECUADOR. Label 1: “ECUADOR: Orellana Province / Yasuni Research Station, / Trap, Canopy - 27 m / Malaise M7-1, AT934 / 11-18.vii.2008, A. Tishechkin”; label 2: “Voucher code M. Reemer / 294 / DNA voucher labcode MZH:Y1089”. Coll. RMNH )preliminary deposition, to be transferred to relevant Ecuadorian collection later’.
In the key of Weems et al. (2003) this species keys to Rhopalosyrphus australis Thompson, 2003 because tergite 3 is short: a little more than half as long as tergite 2. However, in Rhopalosyrphus australis tergite 3 is about 1/3 as long as tergite 2, which places Rhopalosyrphus ecuadoriensis somewhat intermediate between Rhopalosyrphus australis and the other two known species of Rhopalosyrphus, as far as this character is concerned. Other differences with Rhopalosyrphus australis are (character state in Rhopalosyrphus australis in parentheses): antennal ratio approximately as 5:1:8 (5:1:10), mesoscutum almost entirely black pilose (white pilose), tergite 3 black (yellow).
Adult male. Body size: 9 mm.
Head. Face occupying slightly less than 1/3 of head width in frontal view; black, except yellow on lateral 1/4 on dorsal 2/3; golden yellow pilose, most densely at yellow lateral parts. Gena black, yellow pilose. Lateral oral margins produced. Frons black; yellow pilose. Vertex black; yellow pilose, except black pilose posteriad of ocelli. Occiput black; yellow pilose. Eye bare; with narrow, horizontal area frontally at level of antenna with enlarged ommatidia. Antennal fossa about 1.5 times as wide as high. Antenna black; antennal ratio approximately as 5:1:8; longer than distance between antennal fossa and anterior oral margin.
Thorax. Black with faint metallic hues. Mesoscutum black pilose, except narrowly white pilose along anterior margin and with small patches of white pile at notopleuron. Postpronotum and postalar callus white pilose. Scutellum semicircular; black pilose dorsally, white pilose along margins; with small apical calcars, with mutual distance about 1/5 of basal width of scutellum. Anepisternum without sulcus; entirely white pilose, except for small patch of black pile posterodorsally. Anepimeron entirely white pilose. Katepisternum white pilose dorsally, bare ventrally. Katepimeron white pilose anteriorly. Katatergum long microtrichose, anatergum short microtrichose. Calypter and halter yellowish white.
Wing: Hyaline, except faintly infuscated around spur on vein R4+5, vein dm-cu, r-m and bm-cu. Microtrichose, except bare on cell bc, basal 1/2 of cell c, basally on cell r1 along vein Rs, entirely on cell br, basal 3/4 of cell bm, anterobasal 1/2 of cell cup and basomedian 1/10 of alula.
Legs: Front and mid femora blackish brown, except narrowly yellow at apex; white pilose, except for sparse long, black pile posterodorsally. Front and mid tibiae pale yellow basally, dark yellow apically; white pilose, except for sparse black pile postero-apically. Front and mid tarsi yellow; black pilose dorsally, yellow pilose ventrally. Hind femur black; white pilose anteriorly and dorsally, black pilose posteriorly and with dense, bristly to spiny black pile ventrally. Hind tibia pale yellow on basal 3/5; yellow pilose, except black pilose posteriorly at apical 1/4. Hind tarsus brown; black pilose dorsally, yellow pilose ventrally. Coxae and trochanters brown to blackish; white pilose.
Abdomen. Constricted, about as wide as thorax, with narrowest point just before posterior margin of tergite 2. Tergites black with bronze hues, except tergite 2 yellow along posterior margin. Tergite 1 white pilose, except white pilose on median 1/4. Tergite 2 white pilose, except black pilose dorsomedially on apical 1/2. Tergite 3 white pilose, except for dorsomedian triangle of black pile over entire length, which is widest at posterior margin; white pile posterolaterally thicker and more conspicuous, thus forming a medially interrupted fascia at posterior margin. Tergite 4 black pilose, except white pilose along lateral margins and with fascia of golden yellow pile on posterior 1/3, which is partly interrupted by black pile anteromedially. Sternite 1 dark brown; bare. Sternite 2 brown on anterior 2/3, yellow on posterior 1/3; white pilose. Sternite 3 yellow anteriorly and along posterior margin, brown medially; white pilose. Sternite 4 brown; black pilose, except white pilose along posterior margin. Male genitalia as in Fig. 352.
Female. Unknown.
The specific epithet (noun in the genitive case) refers to the country where the type has been collected.
urn:lsid:zoobank.org:act:C36F4365-0BCF-467F-8BD0-35B4F9B28402
http://species-id.net/wiki/Rhopalosyrphus_robustus
Figs 337–341HOLOTYPE. Adult female. FRENCH GUYANA. Label 1: “FRENCH GUYANA. Patawa, / 4°32.658"N-52°9.132"W / VIII.2008. Malaise trap in / pine forest. Leg. O. Morvan”; label 2: “J. Skevington / mol. specimen no / JS3616 - CNC coll.”; label 3: “Voucher code M. Reemer / 271 / DNA voucher labcode MZH:Y1066”. Coll. CNC.
Care should be taken in assessing the presence of pile on the katepimeron: in this species this pilosity is very sparse and limited to the anterior margin. Within Rhopalosyrphus s.s. this species is readily distinguished by the pair of large yellow maculae on tergite 2.
Adult female. Body size: 14.5 mm.
Head. Face occupying about 1/4 of head width in frontal view; black, except yellow on lateral 1/6 on dorsal 2/3; entirely silvery white pilose. Gena black, white pilose. Lateral oral margins produced. Frons black; silvery white pilose. Vertex black; black pilose, except white pilose along eye margin. Occiput black; white pilose, except black pilose dorsolaterally. Eye bare; with narrow, horizontal area frontally at level of antenna where ommatidia are separated from each other by wide spaces; the ommatidia present in this area are larger than elsewhere on the eye. Antennal fossa about 1.5 times as wide as high. Antenna black; antennal ratio approximately as 5:1:9; longer than distance between antennal fossa and anterior oral margin.
Thorax. Black with faint metallic hues. Mesoscutum anteriad of transverse suture appressed white pilose, except for three patches of appressed black pile, and narrowly erect white pilose along anterior margin; mesoscutum posteriad of transverse suture appressed black pilose, except erect white pilose on posterior 1/3. Postpronotum and postalar callus white pilose, except postalar callus laterally black pilose. Scutellum semicircular; white pilose dorsally, golden pilose along margins; without calcars. Anepisternum with shallow sulcus; entirely silvery white pilose. Anepimeron entirely silvery white pilose. Katepisternum silvery white pilose dorsally, bare ventrally. Katepimeron almost bare: with very sparse white pile along anterior margin. Katatergum long microtrichose, anatergum short microtrichose. Calypter and halter yellow.
Wing: Hyaline, faintly infuscated on anterior 1/3. Microtrichose, except bare on cell bc, basal 1/3 of cell r1, entirely on cell br, posterobasally on cell r4+5 posteriad of vena spuria, posterior 1/2 of cell bm, anterior 1/2 of cell cup, and basomedian 3/5 of alula.
Legs: Front femur brown dorsally, black ventrally; white pilose, except for sparse black pile posteriorly. Front tibia black, except brown anteriorly on basal 1/4 and apical 1/3; white pilose, except black pilose ventrally. Front tarsus black, except fifth tarsomere brown; yellow pilose. Mid femur black, except brown anteriorly on basal half; white pilose. Mid tibia black, except yellowish on basal 1/6; white pilose. Mid tarsus black on basal three tarsomeres (other tarsomeres missing in holotype); yellow pilose. Hind femur strongly swollen, about 5 times as wide as mid femur; black; white pilose, except sparsely black pilose dorsally and densely occupied with short, bristly, black pile ventrally. Front coxa and trochanter brown; white pilose. Mid coxa black; white pilose. Mid trochanter brown; white pilose. Hind coxa and trochanter black; white pilose.
Abdomen. Constricted, about as wide as thorax, narrowest just before posterior margin of tergite 2. Tergites black with faint metallic hues, except tergite 2 with pair of large, elongate yellow maculae from anterior 1/4 to posterior 1/3. Tergite 1 white pilose. Tergite 2 white pilose, except for patch of black pile dorsally between middle of tergite and posterior 1/6. Tergite 3 black pilose, except narrowly white pilose along posterior and lateral margins. Tergite 4 pilose as tergite 3, but with sparse yellowish pile intermixed among the black pile on posterior 2/3. Tergite 5 mostly yellowish white pilose, with sparse black pile intermixed anteriorly, and colour of pile more whitish near posterior and lateral margins. Sternite 1 brown; white pilose. Sternite 2 yellow; white pilose. Sternite 3 brown; white pilose. Sternite 4 brown; white pilose, on anterior half, mostly black pilose on posterior half, yellowish pilose along posterior margin. Sternite 5 brown; mixed yellowish and black pilose.
Male. Unknown.
The Latin adjective robustus (strong as oak – Robur) was chosen as the specific epithet because of the size, robustness and stout hind femora of this species, which evoke the impression of a stout fly (strong animal).
urn:lsid:zoobank.org:act:A572ADE8-FBFD-45BD-841E-4AB3A7A41A93
http://species-id.net/wiki/Rhopalosyrphus_abnormoides
Figs 342–345, 353HOLOTYPE. Adult male. PARAGUAY. Label 1: “Paraguay / Fiebrig”; label 2: “S. Bernar- / dino”; label 3: “Myxogaster”; label 4: “Ropalosyrphus [male symbol] / ? auricinctus Sack / det. v. Doesburg”; label 5: “Voucher code M. Reemer / 289”. Coll. RMNH.
Within Rhopalosyrphus s.l. this species is closely related to Rhopalosyrphus abnormis (Curran, 1925). From that species it differs by the following characters (with character state in Rhopalosyrphus abnormis in parentheses): eye bare (pilose); antennal ratio approximately as 4:1:9 (2:1:2.5); scutellum without calcars (with calcars); anterior margin of tergite 2 clearly wider than posterior margin (about as wide).
Adult male. Body size: 11 mm.
Head. Face occupying a little less than 1/3 of head width in frontal view; yellow, with narrow, vaguely defined brown median vitta; entirely golden yellow pilose. Gena black, white pilose. Lateral oral margins produced. Frons black; silvery white pilose. Vertex black; black pilose, except yellow pilose along anterior and lateral margins. Occiput black; black pilose dorsally, golden pilose laterally, silvery white pilose on ventral half. Eye bare; with narrow, horizontal area frontally at level of antenna where ommatidia are separated from each other by wide spaces; the ommatidia present in this area are larger than elsewhere on the eye. Antennal fossa about 1.5 times as wide as high. Antenna black; antennal ratio approximately as 4:1:9; longer than distance between antennal fossa and anterior oral margin.
Thorax. Blackish brown with bronze and green metallic hues. Mesoscutum appressed black pilose, except for fasciae of appressed golden pile along anterior margin, transverse suture and posterior margin. Postpronotum and postalar callus white pilose. Scutellum semicircular; golden pilose; without calcars. Anepisternum with shallow sulcus; golden pilose anterodorsally, silvery white pilose posteriorly, widely bare anteroventrally. Anepimeron entirely silvery white pilose. Katepisternum silvery white pilose dorsally, bare ventrally. Katatergum long microtrichose, anatergum short microtrichose. Calypter and halter yellow.
Wing: Hyaline. Microtrichose, except bare on cell bc, basal 5/6 of cell br, basal 1/6 of cell cup.
Legs: Yellow, except hind femur dark brown and hind tibia medially dark brown. Front and mid legs white to yellow pilose, except mid femur dorsally, anteriorly and ventrally black pilose. Hind leg white to yellow pilose, except femur ventrally densely occupied with short, black, bristly pile. Front coxa orange, mid and hind coxae brown; all coxae white pilose. Front and mid trochanters yellow, hind trochanter brown; all trochanters white pilose.
Abdomen. Constricted, narrower than thorax, with narrowest point halfway tergite 2. Tergites brown with faint metallic hues, except tergite 2 with pair of large rectangular yellow maculae on basal 3/5. Tergite 1 white pilose. Tergite 2 yellow pilose, except sparsely black pilose medially and white pilose along posterior margin. Tergite 3 black pilose, except white pilose anterolaterally and along lateral margin, and with fascia of golden pile along posterior margin; this fascia medially interrupted and gradually narrowing towards lateral margins. Tergite 4 black pilose, except golden pilose anterolaterally and along lateral margin, and with pair of large triangular patches of golden pile over posterior 2/3. Sternite 1 brown; bare. Sternite 2 yellow; short yellow pilose. Sternites 3 and 4 brown; white pilose. Male genitalia as in Fig. 353.
Female. Unknown.
urn:lsid:zoobank.org:act:A17706B2-D5D4-462B-A00A-9166597A8FC8
http://species-id.net/wiki/Rhopalosyrphus_oreokawensis
Figs 347–351, 355HOLOTYPE. Adult male. FRENCH GUYANA. Label 1: “FRENCH GUYANA / Kaw Mountains / 04°32.893"N-52°10.245"W / 27.XI.2002. leg. V. Soon”. Coll. RMNH.
Within Rhopalosyrphus s.l. this species is singular because of its short antenna (slightly shorter than distance between antennal fossa and anterior oral margin) and the shape of the ventral part of the face.
Adult male. Body size: 13 mm.
Head. Face occupying a little more than 1/3 of head width in frontal view; black; entirely white pilose; ventral part of face anteriad of oral margin with lateral bulges, medially separated by shallow, smooth sulcus. Gena black, white pilose. Lateral oral margins slightly produced. Frons, vertex and occiput black; white pilose, except for sparse black pile on frons. Eye bare. Antennal fossa about as high as wide. Antenna brown; antennal ratio approximately as 4:1:3; slightly shorter than distance between antennal fossa and anterior oral margin.
Thorax. Black. Mesoscutum black pilose, except for fasciae of white pile along anterior margin and transverse suture, and patches of white pile posterolaterally. Postpronotum and postalar callus white pilose. Scutellum semicircular; white pilose; with small calcars with mutual distance about equal to 1/4 of width of scutellum. Anepisternum with deep sulcus; black pilose anterodorsally, white pilose posteriorly, widely bare in between. Anepimeron entirely white pilose. Katepisternum white pilose dorsally, bare ventrally. Katatergum long microtrichose, anatergum short microtrichose. Calypter yellow. Halter brown.
Wing: Hyaline, but infuscated at apical 1/2 of cell c, cell sc, around vein r-m, around vein R4+5 and posterior appendix of that vein, around vein dm-cu and around bm-cu. Microtrichose, except bare on cell bc, basal 1/2 of cell c, basal 1/2 of cell r1, basal 1/3 of cell r2+3, posterobasal 1/4 of cell r4+5, entirely bare on cell br, basal 9/10 of cell bm, basal 2/3 of cell cup, and entirely on alula.
Legs: Front and mid legs orange brown, except mid femur blackish brown on basal 2/5; white pilose, except front tibia black pilose on apicodorsal 1/4, tarsi dorsally black pilose and mid femur on apical 1/2 posterodorsally with sparse bristle-like pile among long white pile. Hind leg black, except basal 1/2 of tibia and apical four tarsomeres dark brown; white pilose, except tarsus dorsally black pilose and femur on apical 1/5 with row of short, black, bristle-like pile anteroventrally; femur swollen: about 2.5 times as wide as mid femur. Front coxa brown, mid and hind coxae black; all coxae white pilose. Front and mid trochanters brown, hind trochanter black; all trochanters white pilose.
Abdomen. Constricted, narrower than thorax, with narrowest point at transition between segments 2 and 3. Tergites black, except tergite 2 with pair of large elongate yellow maculae on basal 3/5 and narrowly yellow along posterior margin, and tergite 3 vaguely brownish yellow along anterior margin. Tergite 1 white pilose. Tergite 2 short black pilose, except with long white pile anterolaterally and with thick, appressed white pile along posterior margin. Tergite 3 short black pilose, except for medially interrupted fasciae of thick, appressed white pile along anterior and posterior margins. Tergite 4 yellow pilose on lateral 1/4 and posterior 3/5, black pilose anteromedially and on narrow median vitta on posterior 3/5. Sternite 1 black; bare. Sternite 2 yellow except black along posterior margin; with sparse long white pile. Sternite 3 yellow at anterior 3/5, black posteriorly; short black pilose on posterior 1/2 to 3/5, long, thick white pilose along posterior and posterolateral margins. Sternite 4 brown; black pilose medially, yellow pilose laterally. Male genitalia as in Fig. 355.
Female. Unknown.
The specific epithet (noun in the genitive case) is composed of the Greek oreos (mountain) and Kaw, the name of the French Guyanan mountain region in which the species was found.
This species is very aberrant from other known species of Rhopalosyrphus because of the short antenna, the straight facial profile, the bare katepisternum and the long and slender second abdominal segment. These characters suggest that the species may not belong in Rhopalosyrphus. However, it is certainly related to that genus, considering the structure of the male genitalia and the constricted abdomen. If a new genus were to be erected for this species, more evidence on its phylogenetic affinities to Rhopalosyrphus and other related genera (e.g. Pseudomicrodon) should be available.
urn:lsid:zoobank.org:act:3E0D62D7-5B4C-40B9-A8E6-F20C82361A00
http://species-id.net/wiki/Thompsodon_conspicillifrons
Figs 406–416HOLOTYPE. Female. Label 1: “COSTA RICA, Prov. Limón, / A.C.L.A.C., Talamanca, San Miguel, / Albergue, CASACODE, Send Cerillos. / 10-30 m. 23-26 FEB 1999. M. Lobo. / L_S_391000_612000 #52454”; label 2: “MCR-12”; label 3 (barcode): “INB0003024775 / INBIOCRI COSTA RICA”. Coll. INBIO.
See generic key and genus account of Thompsodon (only one species known).
Adult male. Body size: 8 mm.
Head. Face occupying about 1/3 of head width in frontal view; yellow with black median vitta, which is dorsally about as wide as the antennal fossa and gradually narrows downward, becoming absent in ventral 1/4; yellow pilose, except for sparse black pile submedially, narrowly bare medially. Gena blackish; yellow pilose. Lateral oral margins not produced. Frons black; golden pilose; laterally with round, concave areas, filled with dense golden pile, ventrally delimited by a sharply defined ridge. Vertex irregularly swollen; black; short golden pilose. Ocellar triangle not elevated. Occiput narrow ventrally, strongly widened dorsally; black; golden pilose. Eye bare. Antennal fossa about as high as wide. Antenna with scape pale brown, pedicel and basoflagellomere blackish brown; antennal ratio approximately as 4:1:4.
Thorax. Mesoscutum black; golden pilose, except for pair of black pilose patches anteriad of transverse suture and wide fascia of black pile posteriad of transverse suture. Postpronotum and postalar callus brown, golden pilose. Scutellum black; golden pilose. Pleuron yellowish brown, except anepisternum and anepimerond blackish. Anepisternum with deep sulcus separating posterior from anterior part; entirely mixed yellow and black pilose. Anepimeron entirely mixed yellow and black pilose. Katepisternum long yellow pilose dorsally, bare ventrally. Katatergum short microtrichose, anatergum bare. Calypter dark greyish. Halter yellow.
Wing: hyaline, slightly brownish, especially anteriorly; microtrichose, except bare on cell bc, posterobasal 1/6 of cell c, basal 1/6 of cell r1, entirely on cell br except microtrichose on vena spuria, basal 1/2 of cell bm, and basal 1/2 of cell cup.
Legs: Femora blackish brown, except yellow apically; black pilose. Tibiae and tarsi yellow. Tibiae yellow pilose, except black pilose apically. Tarsi black pilose. Coxae and trochanters blackish brown; black pilose.
Abdomen. More or less oval, but tergite 1 very narrow, so appears constricted basally. Tergites 3 and 4 not fused, able to articulate independently. Tergites blackish. Tergite 1 yellow pilose. Tergite 2 short black pilose, with medially interrupted fascia of longer golden pile along posterior margin. Tergite 3 with similar pattern of pile as tergite 2, but fascia of golden pile medially strongly extended over median part of tergite. Tergite 4 largely golden pilose, except for narrow median vitta of black pile and sublateral oblique vittae of black pile. Tergite 5 golden pilose. Sternites black. Sternite 1 bare, other sternites golden pilose.
Male. Unknown.
The specific epithet (noun in apposition) is composed of the Latin words conspicillum (spectacles) and frons (forehead). The name refers to the concave lateral areas on the frons which (in the eyes of susceptible beholders) evoke the impression of glasses on a forehead.
This species was first recognized as an undescribed taxon by F.C. Thompson, who gave it the preliminary code-name Microdon MCR-12.
In total, 552 species group names (excluding misspellings) applying to Microdontinae are currently known, including 98 synonyms and 26 species described in the present paper. Based on the generic diagnoses and discussions in the preceding section of this paper, the classification of all but a few of these species is re-evaluated. This has resulted in a new species classification, partly based on examination of type material. Primary types (or, in seven of these cases, photographic images of those) of 347 specific taxa were examined. In addition, the classification of six species is based on paratypes. In several cases, no type material was examined, e.g. in the case of well-known taxa from temperate regions, in the case of groups that have been revised by other authors (Mixogaster, Spheginobaccha), in the case of recently described species of which good illustrations are available, and in cases of species of which the types could not be found. For these cases, original descriptions, additional material and literature have been consulted. For each taxon, the source of the information on which the classification was based is indicated (for legend see below).
Of all 552 available species group names, 454 are here considered as valid, 98 as synonyms (19 of which are new synonyms proposed here). Of all available species group names, 267 are presented here in new combinations, while 20 species names (including three synonyms) are left unplaced.
The following format is used:
species name Author, Year: page (Original genus). COLLECTION: KIND_OF_TYPE SEX/STAGE. [SOURCE] Taxonomic act (e.g. comb. n., stat. n.). Remarks.
*: An asterisk denotes information which supplements or corrects information in Systema Dipterorum (Thompson 2010).
Acronyms for type information follow Systema Dipterorum (Thompson 2010):
KIND_OF_TYPE: HT = holotype; LT = lectotype; NT = neotype; ST = syntype(s); T = unspecified.
SEX/STAGE: A = adult; F = female; L = larva; M = male; P = puparium.
[SOURCE]: This indicates the source of the information on which the classification is based. The following codes are used:
1a = primary type(s) studied
1b = photograph(s) of primary type(s) studied
1c = paratype(s) studied
2 = description studied
3 = non-type specimens studied
4 = additional literature studied
Synonymies are based on Thompson (2010), unless they are marked with “Syn. n.”. In the latter case, they are based on the judgement of the first author. Information on the type locality and a full reference to the description is omitted, as this can be found in Thompson (2010) and the regional Diptera catalogues.
Genus Afromicrodon Thompson, 2008
Afrotropical
comoroensis De Meyer, De Bruyn & Janssens, 1990: 571 (Ceratophya). RMCA*: HT* M*. [1a]
johannae Doesburg, 1957: 109 (Ceratophya). MNHN*: HT* M*. [1a] Paratypes in RMNH.
luctiferus Hull, 1941: 320 (Microdon). ANSP: T F*. [3: RMCA] Comb. n.
madecassa Keiser, 1971: 256 (Ceratophya). MNHN*: HT* M*. [1a] Possible junior synonym of Afromicrodon luctiferus (Hull, 1941).
stuckenbergi Keiser, 1971: 258 (Ceratophya). MNHN*: HT* F*. [1a]
Genus Archimicrodon Hull, 1945 stat. n.
Subgenus Archimicrodon Hull, 1945
Afrotropical
ampefyanus Keiser, 1971: 239 (Microdon). MNHN*: HT* M*. [1a] Comb. n.
brevicornis Loew, 1858: 376 (Microdon). NHRS*: ST* MF*. [1a] Comb. n.
caeruleomaculatus Keiser, 1971: 241 (Microdon). MNHN*: HT* F*. [1a] Comb. n.
clatratus Keiser, 1971: 240 (Microdon). MNHN*: HT* F*. [1a] Comb. n.
fenestrellatus Keiser, 1971: 242 (Microdon). MNHN*: HT* M*. [1a] Comb. n.
kavitahaius Keiser, 1971: 243 (Microdon). MNHN*: HT* F*. [1a] Comb. n.
liberiensis Curran, 1929: 4 (Microdon). AMNH: HT F*. [1c] Comb. n. Paratypes (1 male & 1 female) in RMCA.
malagasicus Keiser, 1971: 244 (Microdon). MNHN*: HT* F*. [1a] Comb. n.
nigrocyaneus Hull, 1964: 460 (Microdon). MZLU: HT* F*. [1a] Comb. n.
obesus Herve-Bazin, 1913: 100 (Microdon). RMCA*: HT* M*. [1a] Comb. n. Holotype (male) & allotype (female) in RMCA.
ranavalonae Keiser, 1971: 246 (Microdon). MNHN*: HT* F*. [1a] Comb. n.
sudanus Curran, 1923: 146 (Microdon). BMNH: HT F*. [1a] Comb. n.
tenuifrons Curran, 1929: 5 (Microdon). AMNH: HT M. [1a] Comb. n.
testaceus Walker, 1857: 152 (Microdon). BMNH (lost)*: T A. [3: RMNH & ZMAN] Comb. n.
wainwrighti Curran, 1938: 6 (Microdon). AMNH: HT M*. [1a] Comb. n.
Australian / Oceanian
barringtonensis Ferguson, 1926: 180 (Microdon). ANIC*: HT* M*. [1b] Comb. n.
boharti Curran, 1947: 2 (Microdon). AMNH: HT F. [1a] Comb. n.
brachycerus Knab & Malloch, 1912: 235 (Microdon). USNM*: HT* M. [1a] Comb. n. Type specimen with empty puparium.
browni Thompson, 1968: 44 (Microdon). MCZ: HT M. [1b, 2] Comb. n.
fergusoni Goot, 1964: 220 (Microdon). QMBA*: T M*. [1a] Comb. n. Replacement name for Microdon modestus Ferguson, 1926.
= modestus Ferguson, 1926: 179 (Microdon). Comb. n. Preocc. Knab, 1917.
=fergusoni Thompson, 1968: 44 (Microdon). Comb. n. Preocc. Goot, 1964.
grageti Meijere, 1908: 207 (Microdon). HNHM (lost)*: T M. [2] Comb. n. Type lost (de Jong 2000).
incisuralis Walker, 1865: 113 (Paragus). BMNH: HT* F. [1a] Comb. n.
limbinervis Meijere, 1908: 208 (Microdon). HNHM (lost)*: T F. [3: ZMAN, ID by De Meijere]. Comb. n. Type lost (de Jong 2000). Non-type female identified by de Meijere in ZMAN.
luctiferus Walker, 1865: 113 (Paragus). BMNH: T F. [1a] Comb. n.
malukensis Reemer, sp. n. (Archimicrodon). RMNH: HT M. [1a] Sp.n.
nicholsoni Ferguson, 1926: 173 (Microdon). ANIC*: HT* F. [1b] Comb. n.
novaeguineae Meijere, 1908: 206 (Microdon). ZMAN*: ST* F*. [1a] Comb. n.
purpurescens Shiraki, 1963: 147 (Microdon). USNM: HT F. [2] Comb. n.
tabanoides Hull, 1944: 246 (Microdon). BMNH: HT* F. [1a] Comb. n.
venosus Walker, 1865: 112 (Paragus). BMNH: T M*. [1a] Comb. n.
= papuanus Doesburg, 1959: 234 (Microdon). RMNH: HT* [1a] Syn. n., comb. n.
vittatus Macquart, 1850: 433 (Aphritis). OUMNH*: T F. [1a] Comb. n.
= transiens Walker, 1852: 225 (Eumerus). BMNH: T F. [1a] Comb. n.
= pachypus Bigot, 1884: 541 (Paragus). BMNH: HT* [1a] Comb. n.
Oriental
caeruleus Brunetti, 1908: 92 (Microdon). ZSI*: HT F. [2] Comb. n.
clavicornis Sack, 1926: 592 (Microdon). USNM*: HT* F. [1a] Comb. n.
investigator Hull, 1937: 20 (Microdon). MCZ: HT M. [1b] Comb. n.
lanka Keiser, 1958: 213 (Microdon). NMB Basel: HT* F. [1a] Comb. n.
minuticornis Curran, 1931: 342 (Microdon). BMNH: HT F*. [1c: USNM] Comb. n.
simplicicornis Meijere, 1908: 205 (Microdon). ZMAN: HT* M. [1a] Comb. n.
= digitator Hull, 1937: 19 (Microdon). MCZ: HT M. [1b] Syn. n., comb. n.
varicornis Sack, 1926: 594 (Microdon). USNM*: HT* F. [1a] Comb. n.
Palaearctic
simplex Shiraki, 1930: 15 (Microdon). NIAS: T A. [3: RMNH & coll. M. Hauser] Comb. n. Described as var. of the Oriental Microdon caeruleus Brunetti, 1908.
Subgenus Hovamicrodon Keiser, 1971
Afrotropical
flavifacies Keiser, 1971: 248 (Hovamicrodon). MNHN*: T F*. [1a] Comb. n.
fuscipennis Keiser, 1971: 249 (Hovamicrodon). MNHN*: T F*. [1a] Comb. n.
hova Herve-Bazin, 1913: 398 (Microdon). MNHN*: ST* MF*. [1a] Comb. n.
nubecula Keiser, 1971: 250 (Hovamicrodon). MNHN*: T F*. [1a] Comb. n.
silvester Keiser, 1971: 251 (Hovamicrodon). MNHN*: HT* M*. [1a] Comb. n.
vulpicolor Hull, 1941: 321 (Microdon). ANSP: T F*. [3: RMCA] Comb. n.
Genus Aristosyrphus Curran, 1941
Subgenus Aristosyrphus Curran, 1941
Neotropical
boraceiensis Papavero, 1962: 319 (Ceratophya). MZUSP: HT F. [2]
carpenteri Hull, 1945: 76 (Ceratophya). MCZ: HT F. [1b]
minutus Thompson, 2004: 567 (Aristosyrphus). DZUP: HT* M*. [2]
primus Curran, 1941: 252 (Aristosyrphus). AMNH: HT M. [1a]
Subgenus Eurypterosyrphus Barretto & Lane, 1947
Neotropical
currani Goot, 1964: 214 (Microdon). BMNH*: F*. [1a] Replacement name for Microdon clavicornis Curran, 1940.
= clavicornis Curran, 1940: 6 (Microdon). BMNH: T F. Preocc. Sack, 1926.
macropterus Curran, 1941: 254 (Ceratophya). AMNH: HT F. [1a]
melanopterus Barretto & Lane, 1947: 142 (Eurypterosyrphus). MZUSP: HT F. [1a]
Genus Bardistopus Mann, 1920
Australian / Oceanian
papuanum Mann, 1920: 61 (Bardistopus). USNM: HT M. [1a]
Genus Carreramyia Doesburg, 1966 stat. n.
Neotropical
flava Sack, 1941: 117 (Ceratophya). SNSD*: HT* F*. [1a] Comb. n.
megacephalus Shannon, 1925: 213 (Microdon). USNM: HT M. [1a]
[Descriptions of two additional species from the Neotropical region are in preparation by the first author.]
Genus Ceratophya Wiedemann, 1830
Neotropical
carinifacies Curran, 1934: 376 (Microdon). AMNH: T F. [1a]
notata Wiedemann, 1824: 14 (Ceratophya). NMW*: M*. [1a]
panamensis Curran, 1930: 6 (Microdon). AMNH: HT M. [1a]
scolopus Shannon, 1927: 20 (Microdon). BMNH: T M. [1a] Comb. n.
[The description of an additional species from the Neotropical region is in preparation by the first author.]
Genus Ceratrichomyia Séguy, 1951
Afrotropical
angolensis Reemer sp. n. (Ceratrichomyia). CNC: HT M. [1a] Sp. n.
behara Séguy, 1951: 14 (Ceratrichomyia). MNHN: LT* M*. [1a]
bullabucca Reemer sp. n. (Ceratrichomyia). MNHN: HT M. [1a] Sp. n.
Genus Ceriomicrodon Hull, 1937 stat. n.
Neotropical
petiolatus Hull, 1937: 25 (Ceriomicrodon). USNM: T M*. [1a]
Genus Cervicorniphora Hull, 1945 stat. n.
Australian
alcicornis Ferguson, 1926: 171 (Microdon). ANIC*: HT* M. [1b]
Genus Chrysidimyia Hull, 1937 stat. n.
Neotropical
chrysidimima Hull, 1937: 116 (Chrysidimyia). CM*: M*. [1a]
= chrysidiformis Hull, 1944: 241 (Chrysidimyia). Misspelling of C. chrysidimima.
= granulatus Curran, 1940: 9 (Microdon). BMNH: HT F. [1a] Syn. n., comb. n.
= lazuli Hull, 1944: 241 (Chrysidimyia). BMNH: HT F. [1a] Syn. n.
Genus Domodon Reemer gen. n.
Neotropical
zodiacus Reemer sp. n. (Domodon). RMNH: HT M. [1a] Sp. n.
Genus Furcantenna Cheng, 2008
Oriental
nepalensis Reemer sp. n. (Furcantenna). CNC: HT M. [1a] Sp. n.
yangi Cheng, 2008: 29 (Furcantenna). CASB: HT M. [2]
Genus Heliodon Reemer gen. n.
Oriental
chapini Hull, 1941: 438 (Microdon). USNM: HT M. [1a] Comb. n.
doris Reemer sp. n. (Heliodon). RMNH: HT M. [1a] Sp. n.
elisabeth Keiser, 1958: 211 (Microdon). NMB Basel: HT M. [1a] Comb. n.
elisabethanna Reemer sp. n. (Heliodon). QSBG: HT F. [1a] Sp. n.
gloriosus Hull, 1941: 439 (Microdon). USNM: HT M. [1a] Comb. n.
= aurivesta Hull, 1950: 611 (Microdon). BMNH: HT M. [1a] Syn. n.
klossi Curran, 1931: 343 (Microdon). BMNH: HT M. [1a] Comb. n.
tiber Reemer sp. n. (Heliodon). ZMAN: HT M. [1a] Sp. n.
tricinctus Meijere, 1908: 208 (Microdon). ZMAN: ST* MF*. [1a] Comb. n.
Genus Hypselosyrphus Hull, 1937 stat. n.
Neotropical
amazonicus Reemer, in prep. (Hypselosyrphus). Nom. n. Replacement name for Microdon scutellaris Shannon, 1927.
= scutellaris Shannon, 1927: 20 (Microdon). BMNH: T F. [1a] Comb. n. Preocc. Schummel, 1842
anax Thompson, 1976: 61 (Microdon). Comb. n. Replacement name for Microdon analis Curran, 1940.
= analis Curran, 1940: 3 (Microdon). AMNH: HT M. [1a] Comb. n. Preocc. Macquart, 1842.
corbiculipes Papavero, 1962: 320 (Hypselosyrphus). MZUSP: HT F. [1a]
plaumanni Curran, 1940: 3 (Microdon). AMNH: T F. [1a] Comb. n.
trigonus Hull, 1937: 21 (Hypselosyrphus). MCZ: T M. [1a] Comb. n.
ulopodus Hull, 1944: 34 (Ubristes). CU: T F. [1a] Comb. n.
[Descriptions of five additional species from the Neotropical region are in preparation by the first author.]
Genus Indascia Keiser, 1958
Oriental
brachystoma Wiedemann, 1824: 33 (Ascia). ZMUC: LT M. [1a]
gigantica Reemer sp. n. (Indascia). QSBG: HT M. [1a] Sp. n.
gracilis Keiser, 1958: 223 (Indascia). NMB: HT M. [1a]
spathulata Reemer sp. n. (Indascia). RMNH: HT M. [1a] Sp. n.
Genus Kryptopyga Hull, 1944
Oriental
pendulosa Hull, 1944: 130 (Kryptopyga). BMNH: HT M. [1a]
sulawesiana Reemer sp. n. (Kryptopyga). RMNH: HT M. [1a] Sp. n.
Genus Laetodon Reemer gen. n.
Nearctic
laetoides Curran, 1935: 3 (Microdon). AMNH: HT F. [1a] Comb. n.
laetus Loew, 1864: 74 (Microdon). MCZ (lost): ST MF. [3: USNM] Comb. n.
= scitulus Williston, 1887: 10 (Microdon). USNM: HT M. [4: Thompson 1981b] Comb. n.
solitarius Curran, 1930: 8 (Microdon). AMNH*: HT F*. [1a] Comb. n.
violens Townsend, 1895: 34 (Microdon). SEMC*: T F. [3: USNM] Comb. n.
Neotropical
geijskesi Doesburg, 1966: 80 (Microdon). RMNH*: T M. [1a] Comb. n.
Genus Masarygus Brèthes, 1908
Neotropical
palmipalpus Reemer sp. n. (Masarygus). RMNH: HT M. [1a] Sp. n.
planifrons Brèthes, 1908: 442 (Masarygus). MACN: ST MF*. [1a]
Genus Menidon Reemer gen. n.
Neotropical
falcatus Williston, 1887: 9 (Microdon). USNM: LT M. [3: RMNH & USNM] Comb. n.
= aquilinus Giglio-Tos, 1892: 2 (Microdon). MRSN: HT F. [4: Thompson 2007] Comb. n.
= hondurania Hull, 1940: 247 (Microdon). CNC*: HT* M*. [4: Thompson 2007] Comb. n.
= mellogutta Hull, 1943: 104 (Microdon). BMNH: HT F. [4: Thompson 2007] Comb. n.
Genus Mermerizon Reemer gen. n.
Neotropical
inbio Reemer, sp. n. (Mermerizon). INBIO: HT M. [1a] Sp. n.
Genus Metadon Reemer gen. n.
Afrotropical
aethiopicus Rondani, 1873: 282 (Microdon). MCGD*: T F*. [3: BMNH] Comb. n.
apis Speiser, 1913: 145 (Microdon). Type lost?*: T F*. [2] Comb. n. Type not in NMSA, not in SAMC.
appendiculatus Curran, 1929: 6 (Microdon). AMNH*: HT* M*. [1a] Comb. n. Paratype female in RMCA.
aureomagnificus Hull, 1944: 242 (Microdon). BMNH: HT* M*. [1a] Comb. n.
captum Speiser, 1913: 146 (Microdon). T F*. [3: ZMAN & coll. M. Hauser] Comb. n. Male described by Van Doesburg 1956, but this description seems to apply better to the male of Microdon punctulatus Wiedemann.
erythrocephalus Bezzi, 1915: 130 (Microdon). BMNH: T F*. [1a] Comb. n.
inappendiculatus Curran, 1929: 7 (Microdon). AMNH: HT M. [1a] Comb. n.
inermis Loew, 1858: 376 (Microdon). NHRS*: T M*. [1a] Comb. n.
modesticolor Hull, 1944: 251 (Microdon). BMNH*: HT* M*. [1a] Comb. n.
mydas Bezzi, 1915: 128 (Microdon). BMNH: ST* MF*. [1a] Comb. n.
mynthes Seguy, 1953: 157 (Microdon). MNHN: T M*. [1a] Comb. n.
pallidus Bezzi, 1915: 133 (Microdon). BMNH: ST* MF*. [1a] Comb. n.
punctulatus Wiedemann, 1824: 32 (Microdon). ZMUC*: HT* M*. [1a] Comb. n.
= microtuberculatus Hull, 1964: 459 (Microdon). MZLU: HT* F*. [1a] Syn. n., comb. n. Paratype female also in MZLU.
rugosus Bezzi, 1915: 126 (Microdon). BMNH: HT* M*. [1a] Comb. n.
= concolor Bezzi, 1923: 349 (Microdon). MNHN*: HT* M*. [1a] Comb. n.Described as subspecies of Microdon rugosus Bezzi, 1915.
= fuscus Bezzi, 1921: 21 (Microdon). T M*. [2] Comb. n. Described as var. of Microdon rugosus Bezzi, 1915. Junior primary homonym of Microdon fuscus Meijere, 1908.
= melas Bezzi, 1921: 21 (Microdon). T M*. [2] Comb. n. Described as var. of Microdon rugosus Bezzi, 1915.
= minor Bezzi, 1921: 20 (Microdon). T M*. [2] Comb. n. Described as var. of Microdon rugosus Bezzi, 1915.
Australian / Oceanian
apicalis Walker, 1858: 94 (Microdon). BMNH: T F. [1a] Comb. n.
fulvicornis Walker, 1858: 94 (Microdon). BMNH: HT* F*. [1a] Comb. n.
= tuberculatus Meijere, 1913: 359 (Microdon). ZMAN: T M. [1a] Syn. n., comb. n.
Oriental
achterbergi Reemer sp. n. (Metadon). RMNH: HT F. [1a] Sp. n.
albofascia Hull, 1944: 253 (Microdon). BMNH: HT F. [1a] Comb. n.
annandalei Brunetti, 1907: 13 (Microdon). ZSI: HT M. [3: BMNH & SEMC] Comb. n.
auricinctus Brunetti, 1908: 93 (Microdon). BMNH: HT M*. [1a] Comb. n.
auroscutatus Curran, 1928: 152 (Microdon). BMNH: HT M*. [1a] Comb. n.
=variventris Curran, 1928: 154 (Microdon). BMNH: HT F. [1a] Comb. n. Described as var. of Microdon auroscutatus Curran, 1928.
bicolor Sack, 1922: 272 (Microdon). DEI*: T M*. [1a] Comb. n.
bicoloratus Hull, 1944: 254 (Microdon). BMNH: HT M. [1a] Comb. n.
flavipes Brunetti, 1908: 92 (Microdon). ZSI: HT F. [2] Comb. n.
fulvipes Meijere, 1908: 203 (Microdon). RMNH*: T F. [1a] Comb. n.
=aurilinea Hull, 1944: 258 (Microdon). BMNH: HT M*. [1a] Syn. n., comb. n.
fuscicornis Sasakawa, 1960: 451 (Microdon). T M. [2] Comb. n.
fuscus Meijere, 1908: 204 (Microdon). ZMAN*: T F. [1a] Comb. n.
montis Keiser, 1958: 214 (Microdon). NMB Basel: HT M. [1a] Comb. n.
pendelburyi Curran, 1931: 305 (Microdon). BMNH*: HT* F*. [1a] Comb. n.
pretiosus Curran, 1931: 304 (Microdon). BMNH: HT M. [1a] Comb. n.
robinsoni Curran, 1928: 154 (Microdon). BMNH: HT F. [1a] Comb. n.
ruficaudus Brunetti, 1907: 93 (Microdon). BMNH: HT F. [1a] Comb. n.
rutiliventris Vockeroth, 1975: 371 (Microdon). AMNH*: T F*. [1b] Comb. n. Replacement name for Microdon rufiventris Curran, 1942.
= rufiventris Curran, 1942: 4 (Microdon). BMNH: HT F. [1b] Comb. n. Preocc. Rondani, 1848.
rutilus Keiser, 1952: 173 (Microdon). NMB Basel: HT M. [1a] Comb. n.
sacki Goot, 1964: 220 (Microdon). USNM?*: HT M*. [3: type of var. nigritus Hull] Comb. n. Replacement name for Microdon flavipennis Sack, 1926.
= flavipennis Sack, 1926: 593 (Microdon). USNM?*: T M. [2] Comb. n. Preocc. Curran, 1925.
= nigrita Hull, 1944: 257 (Microdon). BMNH: HT M. [1a] Comb. n. Described as var. of Microdon flavipennis Sack, 1926.
squamipennis Brunetti, 1923: 316 (Microdon). ZSI: HT F. [2] Comb. n.
taprobanicus Keiser, 1958: 212 (Microdon). NMB Basel: HT M. [1a] Comb. n.
wulpii Mik, 1899: 143 (Microdon). RMNH*: T F*. [1a] Comb. n. Replacement name for Microdon apicalis Wulp, 1881.
= apicalis Wulp, 1892: 29 (Microdon). RMNH*: HT* F. Comb. n. Preocc. Walker, 1859. Knutson et al. (1975) erroneously cite publication year as 1881.
= wulpii Brunetti, 1908: 93 (Microdon). RMNH*: T F*. Comb. n. Unnecessary replacement name for Microdon apicalis Wulp, 1881. Preocc. Mik, 1899.
Palaearctic
bifasciatus Matsumura, 1916: 254 (Microdon). SEHU*: T F. [3: RMNH] Comb. n.
brunneipennis Huo, Ren & Zheng, 2007: 398 (Microdon). HU*: HT* F*. [2] Comb. n.
pingliensis Huo, Ren & Zheng, 2007: 401 (Microdon). HU*: HT* M*. [2] Comb. n.
spuribifasciatus Huo, Ren & Zheng, 2007: 403 (Microdon). HU*: HT* M*. [2] Comb. n.
Genus Microdon Meigen, 1803
Subgenus Chymophila Macquart, 1834
Nearctic
fulgens Wiedemann, 1830: 82 (Microdon). ZMHU: LT F. [1a]
= euglossoides Gray, 1832: 779 (Microdon). OUMNH: T A. [4: Thompson et al. 1976]
= splendens Macquart, 1834: 486 (Chymophila). OUMNH: LT M. [1a]
Neotropical
angulatus Hull, 1943: 715 (Microdon). BMNH: T M. [1a] Comb. n.
argentinae Hull, 1937: 18 (Microdon). MCZ: T M. [1b] Comb. n.
aurifacius Hull, 1937: 169 (Microdon). USNM*: M*. [1a] Comb. n.
aurifex Wiedemann, 1830: 85 (Microdon). NMW: T M*. [1a] Comb. n.
= trochilus Walker, 1852: 216 (Microdon). BMNH: T M*. [1a] Comb. n.
barbiellinii Curran, 1936: 6 (Microdon). AMNH: T M. [1a] Comb. n.
bruchi Shannon, 1927: 38 (Microdon). USNM: ST F. [1a] Comb. n.
cyaneiventris Macquart, 1846: 249 (Aphritis). OUMNH: ST* F. [1a] Comb. n.
= cyanoventris Williston, 1886: 310 (Aphritis). Comb. n.Misspelling.
cyaneus Perty, 1833: 186 (Microdon). ZSM*: A. [2] Comb. n.
emeralda Hull, 1943: 719 (Microdon). BMNH: HT* M. [1a] Comb. n.
flavoluna Hull, 1943: 718 (Microdon). BMNH: HT* M. [1a] Comb. n.
histrio Wiedemann, 1830: 83 (Microdon). ZMHU: T F. [1a] Comb. n.
inaequalis Loew, 1866: 40 (Microdon). MCZ (lost)*: T M. [3: USNM] Comb. n.
instabilis Wiedemann, 1830: 83 (Microdon). ZMHU: T F. [1a] Comb. n.
= dives Rondani, 1848: 72 (Aphritis). UTOR*: T M. [2] Comb. n.
limbatus Wiedemann, 1830: 85 (Microdon). ZMHU: T A. [1a] Comb. n.
marceli Curran, 1936: 7 (Microdon). AMNH: T M. [1a] Comb. n.
nero Curran, 1936: 6 (Microdon). AMNH: T M. [1a] Comb. n.
nestor Curran, 1940: 11 (Microdon). AMNH: T M. [1a] Comb. n.
opulentus Bigot, 1883: 319 (Microdon). BMNH*: HT* M*. [1a] Comb. n.
pulcher Williston, 1887: 5 (Microdon). USNM: LT* F. [1a] Comb. n.
shannoni Curran, 1940: 8 (Microdon). AMNH: T F. [1a] Comb. n.
splendens Wiedemann, 1830: 84 (Microdon). NMW: T M. [3: USNM] Comb. n.
stramineus Hull, 1943: 703 (Microdon). BMNH: HT* F. [1a] Comb. n.
superbus Wiedemann, 1830: 82 (Microdon). SMF: HT* F. [1a] Comb. n.
tigrinus Curran, 1940: 11 (Microdon). AMNH: T M. [1a] Comb. n.
willistoni Mik, 1899: 143 (Microdon). AMNH*: HT* M*. [1a] Comb. n. Replacement name for Microdon inermis Williston, 1888.
= inermis Williston, 1888: 258 (Microdon). AMNH: T M. Comb. n. Preocc. Loew, 1858.
Oriental
aenoviridis Curran, 1931: 302 (Microdon). BMNH: HT M. [1a] Comb. n.
baramus Curran, 1942: 3 (Microdon). AMNH*: HT F. [1a] Comb. n.
beatus Curran, 1942: 4 (Microdon). AMNH*: HT F*. [1a] Comb. n.
latiscutellaris Curran, 1931: 341 (Microdon). BMNH: HT F. [1a] Comb. n.
lativentris Meijere, 1921: 52 (Microdon). ZMAN: T M. [1a] Comb. n.
= grandis Curran, 1928: 159 (Microdon). BMNH: HT M. [1a] Syn. n., comb. n.
lundura Curran, 1942: 3 (Microdon). AMNH*: HT M. [1a] Comb. n.
stilboides Walker, 1849: 538 (Microdon). BMNH: HT* M*. [1a] Comb. n.
Palaearctic
katsurai Maruyama & Hironaga, 2004: 174 (Microdon). SEHU: HT M. [2, 3: RMNH] Comb. n.
Subgenus Dimeraspis Newman, 1838
Nearctic
abditus Thompson, 1981: 732 (Microdon). USNM: HT M. [1a] Comb. n.
adventitius Thompson, 1981: 735 (Microdon). USNM: HT M. [1a] Comb. n.
fuscipennis Macquart, 1834: 488 (Ceratophya). OUMNH: LT F. [3: USNM] Comb. n.
= agapenor Walker, 1849: 539 (Microdon). BMNH: ST F. [1a] Comb. n. See Thompson 1981.
= pachystylum Williston, 1887: 8 (Microdon). USNM: HT M. [1a] Comb. n. See Thompson 1981.
globosus Fabricius, 1805: 185 (Mulio). MNHN: LT F. [1a] Comb. n. Original type lost (see Zimsen 1964); lectotype designation by Thompson 1981b.
= albipilis Curran, 1925: 54 (Microdon). CNC: HT M. [4: Thompson 1981b] Comb. n.
= conflictus Curran, 1925: 58 (Microdon). CNC*: LT M. [4: Thompson 1981b4] Comb. n.
= hutchingsi Curran, 1927: 89 (Microdon). CNC: HT F. [4: Thompson 1981b] Comb. n.
= marmoratus Bigot, 1883: 320 (Microdon). BMNH*: ST* MF*. [1a] Comb. n.
= podagra Newman, 1838: 373 (Dimeraspis). BMNH: HT M. [1a] Comb. n.
= pseudoglobosus Curran, 1925: 57 (Microdon). SEMC*: HT* M*. [4: Thompson 1981b] Comb. n.
Neotropical
remotus Knab, 1917: 142 (Microdon). USNM: T M. [4: Thompson 1981a] Comb. n.
= banksi Hull, 1942: 91 (Microdon). MCZ: T F. [4: Thompson 1981a] Comb. n.
Subgenus Megodon Keiser, 1971
Afrotropical
planitarsus Keiser, 1971: 245 (Microdon). MNHN*: HT* M*. [1a]
stuckenbergi Keiser, 1971: 253 (Megodon). MNHN*: HT* M*. [1a] Comb. n.
Subgenus Microdon Meigen, 1803 s.s.
Nearctic
abstrusus Thompson, 1981: 735 (Microdon). USNM*: HT M. [1a]. Paratype male in BMNH.
albicomatus Novak, 1977: 664 (Microdon). WSU: HT M*. [3: ZMAN]
aurulentus Fabricius, 1805: 185 (Mulio). MNHN: LT F. [1a]
cothurnatus Bigot, 1883: 320 (Microdon). BMNH*: HT* M*. [1a]
= cockerelli Jones, 1922: 17 (Microdon). USNM: ST M. [4: Thompson 1981b]
lanceolatus Adams, 1903: 222 (Microdon). SEMC: HT M*. [3: USNM]
= coloradensis Cockerell & Andrews, 1916: 53 (Microdon). USNM: HT M*. [1a]
= modestus Knab, 1917: 139 (Microdon). USNM: HT M*. [1a]
= senilis Knab, 1917: 139 (Microdon). USNM: HT F*. [1a]
= similis Jones, 1917: 219 (Microdon). USNM: LT F*. [1a] Described as var. of Microdon cothurnatus Bigot, 1883.
manitobensis Curran, 1924: 227 (Microdon). CNC: LT M*. [1a]
megalogaster Snow, 1892: 34 (Microdon). SEMC: HT M*. [3: BMNH & RMNH]
= bombiformis Townsend, 1895: 33 (Microdon). SEMC: HT F*. [4: Thompson 1981b]
newcomeri Mann, 1924: 94 (Microdon). USNM: HT M*. [1a]
ocellaris Curran, 1924: 227 (Microdon). USNM: LT F*. [1a]
piperi Knab, 1917: 136 (Microdon). USNM: LT M*. [1a]. Holotype with empty puparium.
ruficrus Williston, 1887: 7 (Microdon). USNM: HT M*. [1a] Described as var. of Microdon tristis Loew, 1864.
= basicornis Curran, 1925: 79 (Microdon). CNC: HT M*. [4: Thompson 1981b]
= champlaini Curran, 1925: 71 (Microdon). USNM: HT M*. [1a]
tristis Loew, 1864: 73 (Microdon). MCZ (lost)*: T F*. [3: CNC, RMNH, USNM]
= robusta Telford, 1939: 14 (Microdon). UMSP: HT F*. [4: Thompson 1981b]
xanthopilis Townsend, 1895: 611 (Microdon). SEMC: LT M*. [3: USNM]
Neotropical
aureopilis Marinoni, 2004: 569 (Microdon). CNC: HT* M*. [2]
barbouri Hull, 1942: 89 (Microdon). MCZ: T F. [1b]
bassleri Curran, 1940: 10 (Microdon). AMNH: T F. [1a]
bonariensis Lynch Arribalzaga, 1891: 194 (Microdon). MACN: HT F. [2]
brutus Hull, 1944: 37 (Microdon). CU: T M. [1a]
caesar Curran, 1940: 10 (Microdon). AMNH: T M. [1a]
crassitarsis Macquart, 1848: 198 (Aphritis). OUMNH: HT* M. [1a]
eutristis Curran, 1925: 74 (Microdon). SEMC: T M. [2]
macquartii Lynch Arribalzaga, 1891: 126 (Microdon). OUMNH*: HT* F*. [1a] Replacement name for Aphritis angustus Macquart, 1848.
= angustus Macquart, 1848: 198 (Aphritis). OUMNH*: HT* F*. [1a] Preocc. Macquart, 1846.
= angustatus Fluke, 1957: 29 (Microdon). Misspelling of Microdon angustatus (Macquart, 1848).
mourei Marinoni, 2004: 569 (Microdon). CNC: HT* M*. [2]
remus Curran, 1941: 250 (Microdon). AMNH: T F. [1a]
rufiventris Rondani, 1848: 73 (Aphritis). MZUN: T F. [3: BMNH & RMNH]
violaceus Macquart, 1842: 13 (Aphritis). MNHN: ST* M*. [1a] The description by Macquart (1842) was based on a male from Chile, collected by M. Gay, which corresponds with the data on the label of a specimen in the Macquart collection of the MNHN. There is also a female in the same collection, but without a data label. There are also 12 specimens among the Macquart material in the OUMNH, but these too are without data labels (pers. comm. Z. Simmons).
virgo Curran, 1940: 7 (Microdon). AMNH: T M. [1a]
Oriental
aeneus Keiser, 1952: 172 (Microdon). NMB*: HT* M*. [1a]
alboscutatus Curran, 1931: 303 (Microdon). AMNH*: HT M*. [1a]. There is a specimen labelled as ‘holotype’ in the BMNH-collection, but locality information of that specimen is not correct. The real holotype is in AMNH.
bellus Brunetti, 1923: 315 (Microdon). BMNH: HT F. [1a]
formosanus Shiraki, 1930: 22 (Microdon). NIAS: ST* MF*. [2]
fulvopubescens Brunetti, 1923: 313 (Microdon). BMNH: HT F. [1a]
fumipennis Hull, 1944: 259 (Microdon). BMNH: HT M. [1a]
metallicus Meijere, 1904: 98 (Microdon). ZMAN: T M. [1a]
sumatranus Wulp, 1892*: 29 (Microdon). RMNH*: HT* F. [1a]. Publication year wrong in Knutson et al. 1975.
sumbanus Keiser, 1952: 174 (Microdon). NMB: HT F. [1a]
Palaearctic
analis Macquart, 1842: 72 (Aphritis). MNHN: HT* M*. [1a]
= ?ammerlandia Spix, 1824: 124 (Scutelligera). L. [2] Syn. n. Described as a mollusc.
= brevicornis Egger, 1862: 783 (Microdon). NMW: ST B. [4: Doczkal and Schmid 1999] Preocc. Loew, 1857.
= eggeri Mik, 1897: 66 (Microdon). NMW: T A. [3: several coll.] Replacement name for Microdon brevicornis Egger, 1862.
= fuscitarsis Schummel, 1842: 115 (Microdon). Lost*: T A [4: Doczkal and Schmid 1999]
= latifrons Loew, 1856: 599 (Microdon). Lost*: T A. [4: Doczkal and Schmid 1999]
= ?reticulata Torrez Minguez, 1924: 108 (Buchanania). HT* L*. [2] Syn. n. Described as a mollusc.
auricomus Coquillett, 1898: 320 (Microdon). USNM: HT* M*. [1a]
devius Linnaeus, 1761: 446 (Musca). Lost: T A. [3: several coll.]
= anthinus Meigen, 1822: 165 (Microdon). MNHN: HT M. [4: Doczkal and Schmid 1999]
= conica Panzer, 1793: 21 (Stratiomys). SNSD?: T A. Musca devius Linnaeus, 1761 [4: Doczkal and Schmid 1999]
= micans Wiedemann in Meigen, 1822: 165 (Microdon). MNHN: ST A [4: Doczkal and Schmid 1999]
= picticornis Mik, 1897: 66 (Microdon). A. [4: Doczkal and Schmid 1999] Described as var. of Microdon devius (Linnaeus, 1761).
= pigra Schrank, 1803: 97 (Stratiomys). A. [4: Doczkal and Schmid 1999]
= viridescens Villers, 1789: 463 (Musca). Coll. Villers: T A. [4: Doczkal and Schmid 1999]
hauseri Reemer sp. n. (Microdon). CSCS: HT M. [1a] Sp. n.
ignotus Violovitsh, 1976: 160 (Microdon). ZISP: HT M. [2]
japonicus Yano, 1915: 5 (Microdon). T A. [3: BMNH & RMNH]
= jezoensis Matsumura, 1916: 255 (Microdon). NIAS: ST F [3: BMNH]
kidai Hironaga & Maruyama, 2004: 90 (Microdon). SEHU: HT M. [2]
lateus Violovitsh, 1976: 160 (Microdon). A. [4: Doczkal and Schmid 1999]
lehri Mutin, 1999: 360 (Microdon). HT* M*. [2]
macrocerus Hironaga & Maruyama, 2004: 88 (Microdon). NSMT, Toyko: HT M. [2]
major Andries, 1912: 307 (Microdon). ZFMK*: NT* P*. [3, 4: Schmid 2004]
mandarinus Reemer sp. n. (Microdon). CSCS: HT M. [1a] Sp. n.
maritimus Violovitsh, 1976: 161 (Microdon). ZISP: HT M. [3: USNM]
miki Doczkal & Schmid, 1999: 48 (Microdon). SMNS*: HT* M*. [2] Paratypes in SMNS and ZMHU.
murayamai Hironaga & Maruyama, 2004: 97 (Microdon). SEHU: HT M. [2]
mutabilis Linnaeus, 1758: 592 (Musca). BMNH: LT F. [3: several coll.]
= apiarius Fabricius, 1805: 46 (Mulio). Lost: T A. [4: Doczkal and Schmid 1999] Type lost, only name label remains (Zimsen 1964).
= apiformis De Geer, 1776: 128 (Musca). NHRS: T A. [4: Doczkal and Schmid 1999]
= auropubescens Latreille, 1805: 358 (Aphritis). T A. [4: Doczkal and Schmid 1999]
= ?cocciformis von Heyden, 1825: 589 (Parmula). HT* L*. [2] Described as mollusc.
= rhenanus Andries, 1912: 307 (Microdon). ZFMK*: LT* P*. [4: Schmid 2004]
= scutellatus Schummel, 1842: 116 (Microdon). [4: Doczkal and Schmid 1999]
myrmicae Schönrogge, Barr, Wardlaw, Napper, Gardner, Breen, Elmes & Thomas, 2002: 315 (Microdon). BMNH: HT F. [3: several coll.]
mysa Violovitsh, 1971: 62 (Microdon). ZISP: HT M. [2]
nigripes Shiraki, 1930: 22 (Microdon). NIAS: ST MF. [2] Described as var. of Microdon auricomus Coquillett, 1898.
nigrodorsatum Mutin, 2011: 19 (Microdon). SEHU: HT M. [2]
novus Schrank, 1776: 93 (Musca). A. [4: Peck 1988]
oitanus Shiraki, 1930: 18 (Microdon). NIAS: HT F. [2]
podomelainum Huo, Ren & Zheng, 2007: 402 (Microdon). HU*: HT* F*. [2]
ursitarsis Stackelberg, 1926: 90 (Microdon). A. [2]
yokohamai Hironaga & Maruyama, 2004: 94 (Microdon). HUS: HT M. [2]
yunnanensis Reemer sp. n. (Microdon). CSCS: HT M. [1a] Sp. n.
Subgenus Myiacerapis Hull, 1949
Afrotropical
villosus Bezzi, 1915: 135 (Microdon). BMNH: HT* M*. [1a]
Subgenus Syrphipogon Hull, 1937
Neotropical
fucatissimus Hull, 1937: 120 (Syrphipogon). CM: HT M. [1a]
gaigei Steyskal, 1953: 1 (Microdon). MZM: HT F. [2]
Species groups of Microdon s.l.
craigheadii-group
Nearctic
craigheadii Walton, 1912: 463 (Microdon). USNM: HT M. [1a]
erythros-group
Afrotropical
erythros Bezzi, 1908: 382 (Microdon). T M*. [3: RMCA & RMNH]
= erytherus Bezzi, 1921: 21 (Microdon). M*. Misspelling.
luteiventris Bezzi, 1915: 132 (Microdon). BMNH: ST* MF*. [1a]
mirabilis-group
Neotropical
bertonii Bezzi, 1910: 319 (Microdon). MCSN: ST* MF*. [1a]
= arcuata Curran, 1941: 250 (Microdon). AMNH: T M. [1a] Syn. n.
iheringi Bezzi, 1910: 320 (Microdon). MCSN: LT* M. [1a] For lectotype designation see Genus accounts.
mirabilis Williston, 1888: 257 (Microdon). AMNH: ST* MF*. [1a]
tarsalis-group
Afrotropical
tarsalis Hervé-Bazin, 1913: 98 (Microdon). RMCA*: HT* M*. [1a] Holotype in RMCA, not in MNHN. See also Hervé-Bazin (1913: 69).
= bequaerti Curran, 1929: 3 (Microdon). AMNH: HT M. [1a] Syn. n. Paratype female in RMCA.
Unplaced species of Microdon s.l.
Afrotropical
tsara Keiser, 1971: 247 (Microdon). MNHN*: HT* M*. [1a]
Australian / Oceanian
amabilis Ferguson, 1926: 175 (Microdon). QMBA: T F. [3: CNC]
macquariensis Ferguson, 1926: 174 (Microdon). ANIC*: HT* M. [1b, 3: USNM]
nigromarginalis Curran & Bryan, 1926: 132 (Microdon). ANIC*: HT* F*. [1b, 3: RMNH]
pictipennis Macquart, 1850: 433 (Aphritis). MNHNP: HT* F. [1a]
= pictulipennis Hull, 1944: 249 (Microdon). BMNH: HT* M. [1a] Syn. n.
rieki Paramonov, 1957: 815 (Microdon). ANIC*: HT* M. [1b, 1c: USNM]
waterhousei Ferguson, 1926: 174 (Microdon). AMS: T F. [3: coll. M. Hauser]
Oriental
carbonarius Brunetti, 1923: 314 (Microdon). ZSI: HT M. [1c] Paratype and three additional specimens in BMNH.
pagdeni Curran, 1942: 6 (Microdon). AMNH*: HT F. [1a] Type not found in BMNH. Specimen labelled as such in AMNH.
trimacula Curran, 1928: 156 (Microdon). BMNH: ST* M. [1a]
unicolor Brunetti, 1915: 255 (Microdon). ZSI*: HT M. [2]
Genus Mixogaster Macquart, 1842
Nearctic
breviventris Kahl, 1897: 137 (Mixogaster). CU: F*. [2]
delongi Johnson, 1926: 301 (Mixogaster). MCZ: A. [2]
johnsoni Hull, 1941: 162 (Mixogaster). CNC: HT* A. [2]
Neotropical
anthermus Walker, 1849: 547 (Ascia). BMNH: ST* M*. [1a]
cicatrix Hull, 1954: 9 (Mixogaster). CU: T M. [2]
conopsoides Macquart, 1842: 14 (Mixogaster). MNHN: T F. [1a]
= conopoides Kertesz, 1910: 351 (Mixogaster). A. [2] Emendation.
=conopseus Williston, 1886: 309 (Mixogaster). A. [2] Misspelling.
cubensis Curran, 1932: 1 (Mixogaster). AMNH: T M. [1a]
currani Hull, 1954: 5 (Mixogaster). AMNH: T M. [1a]
dimidiata Giglio-Tos, 1892: 1 (Mixogaster). MRSN: HT F. [2]
= dimitiata Fluke, 1957: 37 (Mixogaster). A. [2] Misspelling.
flukei Hull, 1954: 15 (Mixogaster). AMNH: T M. [1a]
imitator Thompson, 2004: 572 (Mixogaster). USNM: HT* M*. [1c: BMNH]
lanei Carrera & Lenko, 1958: 473 (Mixogaster). MZUSP: T M. [2]
lopesi Carrera & Lenko, 1958: 477 (Mixogaster). MZUSP: T M. [2]
mexicana Macquart, 1846: 251 (Mixogaster). MRHNB: T F*. [2]
orpheus Hull, 1944: 36 (Mixogaster). MCZ: T F. [2]
pithecofascia Hull, 1944: 512 (Mixogaster). CNC: HT M. [2]
polistes Hull, 1954: 4 (Mixogaster). AMNH: T F. [1a]
rarior Shannon, 1925: 111 (Mixogaster). USNM: T M. [1a]
= rarissima (var.) Shannon, 1925: 111 (Mixogaster). USNM*: M*. [1a] Described as var. of Mixogaster rarior Shannon, 1925.
sartocrypta Hull, 1954: 8 (Mixogaster). AMNH: T F. [1a]
strictor Hull, 1941: 1 (Mixogaster). AMNH: T M. [1a]
thecla Hull, 1954: 6 (Mixogaster). AMNH: T F. [1a]
Genus Oligeriops Hull, 1937 stat. n.
Australian / Oceanian
chalybeus Ferguson, 1926: 176 (Microdon). Coll. Hardy: T M. [2] Comb. n.
dimorphon Ferguson, 1926: 177 (Microdon). ANIC*: HT* A. [1b] Comb. n.
iridomyrmex Shannon, 1927: 85 (Microdon). BMNH: ST* F. [1a] Comb. n.
moestus Ferguson, 1926: 518 (Microdon). ANIC: T F. [2] Comb. n.
occidentalis Ferguson, 1926: 176 (Microdon). SAMA: T F. [2] Comb. n.
Genus Omegasyrphus Giglio-Tos, 1891
Nearctic
baliopterus Loew, 1872: 86 (Microdon). MCZ (lost)*: ST MF*. [3: USNM]
= brunnipennis Hull, 1944: 400 (Microdon). NMW: HT M. [2] Described as var. of Microdon baliopterus Loew, 1872.
coarctatus Loew, 1864: 74 (Microdon). MCZ (lost)*: ST MF*. [3: USNM]
gracilis Bigot, 1883: 320 (Microdon). BMNH*: HT* M*. [1a] Comb. n.
painteri Hull, 1922: 370 (Microdon). CNC: HT M*. [1b]
pallipennis Curran, 1925: 89 (Microdon). SEMC: ST A. [3: USNM]
Genus Paragodon Thompson, 1969
Neotropical
paragoides Thompson, 1969: 81 (Paragodon). CNC: HT M. [2]
Genus Paramicrodon Meijere, 1913
Australian / Oceanian
lorentzi Meijere, 1913: 360 (Paramicrodon). ZMAN: T F. [1a]
toxopei Meijere, 1929: 410 (Paramicrodon). ZMAN: T M. [1a]
Neotropical
delicatulus Hull, 1937: 24 (Paramicrodon). MCZ: T M. [1a]
flukei Curran, 1936: 2 (Paramicrodon). AMNH: T M. [1a]
Oriental
cinctellus Sack, 1926: 590 (Myxogaster). DEI?*: T F. [2]
miranda Herve-Bazin, 1926: 74 (Syrphinella). MNHN*: HT* F*. [1a]
nigripennis Sack, 1922: 275 (Myxogaster). T M. [2]
novus Hull, 1937: 22 (Paramicrodon). MCZ: T F. [2]
Genus Paramixogaster Brunetti, 1923
Afrotropical
acantholepidis Speiser, 1913: 141 (Microdon). NMSA*: HT* M*. [1a] Comb. n.
crematogastri Speiser, 1913: 143 (Microdon). NMSA*: HT* F*. [1a] Comb. n.
elisabethae Keiser, 1971: 254 (Pseudomicrodon). MNHN*: T F*. [1a] Comb. n.
illucens Bezzi, 1915: 121 (Microdon). BMNH: T M*. [1a] Comb. n.
piptotus Reemer sp. n. (Paramixogaster). MNHN: HT M. [1a] Sp. n.
Australian / Oceanian
aphritinus Thomson, 1869: 491 (Mixogaster). NHRS: HT* M*. [1a] Stat. n., comb. n.
daveyi Knab & Malloch, 1912: 233 (Microdon). USNM: T F. [1a] Comb. n.
gayi Paramonov, 1957: 814 (Microdon). ANIC*: HT* F. [1b] Comb. n.
odyneroides Meijere, 1908: 213 (Microdon). HNHM (lost)*: T A. [2, 4: Sack 1926] Comb. n.Type lost (de Jong 2000).
omeanus Paramonov, 1957: 813 (Microdon). ANIC: HT* F. [1b, 1c: USNM] Comb. n.
petiolata Hull, 1944: 248 (Microdon). BMNH: HT* F. [1a] Comb. n.
praetermissus Ferguson, 1926: 182 (Microdon). SAMA?: T F. [2] Comb. n.
variegatus Walker, 1852: 220 (Ceratophya). BMNH*: F*. [1a] Comb. n.
wegneri Keiser, 1964: 84 (Paramixogaster). NMB Basel*: HT* M*. [2]
Oriental
brunettii Reemer, sp. n. Nom. n. New replacement name for Mixogaster vespiformis Brunetti, 1913.
= vespiformis Brunetti, 1913: 169 (Mixogaster). ZSI: HT M*. [2] Preocc. De Meijere, 1908.
contractus Brunetti, 1923: 310 (Microdon). BMNH: HT F. [1a] Comb. n.
conveniens Brunetti, 1923: 311 (Microdon). BMNH: HT F. [1a] Comb. n.
decipiens Meijere, 1917: 242 (Paramicrodon). ZMAN*: HT* F*. [1a] Comb. n. Puparia also in ZMAN.
fujianensis Cheng, 695 (Paramixogaster). CASB: HT M. [2]
icariiformis Pendlebury, 1927: 38 (Paramixogaster). BMNH (lost)*: T F. [2] Type not in BMNH.
indicus Doleschall, 1857: 404 (Ceratophya). HNHM (lost)*: T A. [2] Comb. n.
luxor Curran, 1931: 306 (Microdon). BMNH: HT M. [1a] Comb. n.
sacki Nom. n., comb. n. New replacement name for Myxogaster variegata Sack, 1922.
= variegata Sack, 1922: 274 (Myxogaster). F*. [2] Comb. n. Preocc. Walker, 1852.
vespiformis Meijere, 1908: 210 (Microdon). ZMAN*: ST* F*. [1a] Comb. n.
wegneri Keiser, 1964: 84 (Paramixogaster). NMB Basel*: HT* F*. [2]
yunnanensis Cheng, 2012: 696 (Paramixogaster). CASB: HT M. [2]
Genus Parocyptamus Shiraki, 1930
Oriental
sonamii Shiraki, 1930: 12 (Parocyptamus). NIAS*: ST M. [1a]
= purpureus Hull, 1937: 26 (Stenomicrodon). CNC*: HT F. [1b] Syn. n.
stenogaster Curran, 1931: 344 (Microdon). BMNH: HT M*. [1a] Comb. n.
Genus Peradon Reemer gen. n.
Neotropical
bidens-group
angustiventris Macquart, 1855: 105 (Aphritis). OUMNH: HT* M. [1a] Comb. n.
angustus Macquart, 1846: 250 (Aphritis). MNHN (lost)*: T M. [2] Comb. n. Type not found in MNHN.
aurifascia Hull, 1944: 245 (Microdon). BMNH: HT* M. [1a] Comb. n.
bidens Fabricius, 1805: 185 (Mulio). UZMC: HT* M. [1a] Comb. n.
=bicolor Walker, 1857: 151 (Ceratophya). BMNH: HT* F*. [1a] Syn. n., comb. n.
bispina Hull, 1943: 707 (Microdon). BMNH: HT* M. [1a] Comb. n.
elongata Hull, 1943: 706 (Microdon). BMNH: HT M. [1a] Comb. n.
flavipennis Curran, 1925: 342 (Microdon). MCZ: T F. [1b] Comb. n.
flavomarginatum Curran, 1925: 245 (Microdon). CU: T M. [1a] Comb. n.
langi Curran, 1925: 341 (Microdon). AMNH*: T M. [1a] Comb. n.
luridescens Walker, 1857: 151 (Ceratophya). BMNH: T F. [1a] Comb. n.
niger Williston, 1891: 4 (Microdon). BMNH: HT* M. [1a] Comb. n.
=manni Shannon, 1923: 80 (Microdon). USNM: T F. [1a] Comb. n.
normalis Curran, 1925: 343 (Microdon). AMNH: T F. [1a] Comb. n.
oligonax Hull, 1944: 35 (Microdon). CU: T F. [1a] Comb. n.
flavofascium-group
aurigaster Hull, 1941: 160 (Microdon). MCZ: T M. [1b] Comb. n.
chrysopygus Giglio-Tos, 1892: 1 (Ubristes). MRSN*: HT* F*. [1b] Comb. n.
flavofascium Curran, 1925: 346 (Microdon). CU: T M. [1a] Comb. n.
trivittatus-group
aureoscutus Hull, 1943: 709 (Microdon). BMNH: HT* M. [1a] Comb. n.
aureus Hull, 1944: 35 (Microdon). MCZ: T F. [1b] Comb. n.
diaphanus Sack, 1921: 146 (Microdon). DEI: T M. [3: USNM] Comb. n.
fenestratus Hull, 1943: 712 (Microdon). BMNH: HT* M. [1a] Comb. n.
hermetia Curran, 1936: 3 (Microdon). AMNH: HT* M. [1a] Comb. n.
hermetoides Curran, 1940: 8 (Microdon). BMNH: HT* M. [1a] Comb. n.
trilinea Hull, 1943: 710 (Microdon). BMNH: HT* M. [1a] Comb. n.
trivittatus Curran, 1925: 344 (Microdon). AMNH: T M. [1a] Comb. n.
Genus Piruwa Reemer gen. n.
Neotropical
phaecada Reemer sp. n. (Piruwa). RMNH: HT M. [1a] Sp. n.
Genus Pseudomicrodon Hull, 1937
Neotropical
auricinctus Sack, 1931: 148 (Rhopalosyrphus). DEI*: F*. [3: RMNH & USNM] Comb. n.
batesi Shannon, 1927: 22 (Microdon). BMNH: HT* F. [1a] Comb. n.
beebei Curran, 1936: 4 (Microdon). AMNH: T F. [1a]
bellulus Williston, 1891: 1 (Mixogaster). BMNH: HT* M. [1a]
biluminiferus Hull, 1944: 399 (Microdon). NMW: T M. [1a] Comb. n.
chrysostypus Thompson, 2004: 571 (Microdon). USNM: HT* M*. [2] Comb. n.
claripennis Hine, 1914: 334 (Mixogaster). OHSU: T M. [2] Comb. n.
conops Curran, 1940: 4 (Microdon). AMNH: T M. [1a] Comb. n.
corona Curran, 1940: 9 (Microdon). AMNH: T M. [1a] Comb. n.
nigrispinosus Shannon, 1927: 21 (Microdon). BMNH: ST* M. [1a]
pilosops Marinoni, 2004: 572 (Microdon). BMNH: HT* M*. [1a]
polistoides Reemer sp. n. (Pseudomicrodon). RMNH: HT F. [1a] Sp. n.
rheochryssus Hull, 1944: 38 (Microdon). CU: T M. [2] Comb. n.
seabrai Papavero, 1962: 317 (Pseudomicrodon). Seabra*: T M. [2]
smiti Reemer sp. n. (Pseudomicrodon). RMNH: HT M. [1a] Sp. n.
Genus Ptilobactrum Bezzi, 1915
Afrotropical
neavei Bezzi, 1915: 137 (Ptilobactrum). BMNH: HT* M*. [1a]
Genus Rhoga Walker, 1857
Neotropical
lutescens Walker, 1857: 157 (Rhoga). BMNH (lost)*: T F. [2] Type not present in BMNH, probably lost.
maculata Shannon, 1927: 21 (Microdon). BMNH: HT F*. [1a]
mellea Curran, 1940: 5 (Microdon). BMNH: T M. [1a]
sepulchrasilva Hull, 1937: 28 (Papiliomyia). NMW: T M. [1a]
xanthoprosopa Barretto & Lane, 1947: 145 (Rhoga). MZUSP: HT M. [2]
Genus Rhopalosyrphus Giglio-Tos, 1891
Sensu stricto
Nearctic
ramulorum Weems & Deyrup, 2003: 189 (Rhopalosyrphus). USNM: HT M. [1c: BMNH]
[Rhopalosyrphus guentherii, a mainly Neotropical species, is also known from southern states of the USA. However, it is only listed among the Neotropical species below so as to avoid species being mentioned more than once.]
Neotropical
australis Thompson, 2003: 188 (Rhopalosyrphus). AMNH: HT F. [1c: BMNH]
ecuadoriensis Reemer sp. n. (Rhopalosyrphus). RMNH: HT M. [1a] Sp. n.
guentherii Lynch Arribalzaga, 1891: 195 (Holmbergia). MACN: T A. [3: RMNH & USNM]
= carolae Capelle, 1956: 174 (Rhopalosyrphus). SEMC: HT F. [4: Weems et al. 2003]
robustus Reemer sp. n. (Rhopalosyrphus). CNC: HT M. [1a] Sp. n.
Sensu lato (Neotropical)
abnormis Curran, 1925: 345 (Microdon). MCZ: HT* F. [1b] Comb. n.
abnormoides Reemer sp. n. (Rhopalosyrphus). RMNH: HT M. [1a] Sp. n.
cerioides Hull, 1943: 716 (Microdon). BMNH: T M. [1a] Comb. n.
oreokawensis Reemer sp. n. (Rhopalosyrphus). RMNH: HT F. [1a] Sp. n.
Genus Schizoceratomyia Carrera, Lopes & Lane, 1947
Neotropical
barretoi Carrera, Lopes & Lane, 1947: 245 (Schizoceratomyia). MZUSP*: M*. [1a]
carrerai Papavero, 1962: 324 (Masarygus). MZUSP: HT M. [1a]
flavipes Carrera, Lopes & Lane, 1947: 247 (Schizoceratomyia). MZUSP: HT M. [1a]
malleri Curran, 1947: 1 (Johnsoniodon). AMNH: HT M*. [1a]
Genus Serichlamys Curran, 1925 stat. n.
Nearctic
?diversipilosus Curran, 1925: 76 (Microdon). SEMC: HT M. [2] Comb. n.
rufipes Macquart, 1842: 71 (Aphritis). OUMNH: HT* M*. [1a]
= limbus Williston, 1887: 8 (Microdon). USNM: HT F*. [4: Thompson 1981b]
scutifer Knab, 1917: 141 (Microdon). USNM: HT F*. [3: RMNH & USNM] Comb. n.
Neotropical
mitis Curran, 1940: 7 (Microdon). AMNH: T M. [1a] Comb. n.
mus Curran, 1936: 5 (Microdon). AMNH: T M. [1a] Comb. n.
Genus Spheginobaccha de Meijere, 1908
Afrotropical
dexioides Hull, 1944: 131 (Spheginobaccha). BMNH*: M*. [1a]
dubia Thompson, 1974: 280 (Spheginobaccha). NMSA*: HT* M*. [2]
guttula Dirickx, 1995: 155 (Spheginobaccha). MNHN*: M*. [1a]
perialla Thompson, 1974: 284 (Spheginobaccha). BMNH*: HT* M*. [2]
rotundiceps Loew, 1858: 376 (Ocyptamus). NHRS*: HT* F*. [4: Thompson 1974]
= funeralis Hull, 1944: 131 (Spheginobaccha). BMNH*: HT* M*. [4: Thompson 1974]
ruginosa Dirickx, 1995: 152 (Spheginobaccha). MNHN*: F*. [1a]
Oriental
aethusa Walker, 1849: 559 (Xylota). BMNH*: HT* F*. [4: Thompson 1974]
chillcotti Thompson, 1974: 274 (Spheginobaccha). CNC*: HT* M*. [2]
demeijerei Doesburg, 1968: 161 (Spheginobaccha). RMNH*: HT* M*. [1a]
duplex Walker, 1857: 18 (Syrphus). BMNH*: HT* M*. [4: Thompson 1974]
humeralis Sack, 1926: 571 (Doros). USNM*: LT* M*. [1a]
knutsoni Thompson, 1974: 271 (Spheginobaccha). USNM*: HT* F*. [2]
lieftincki Doesburg, 1968: 160 (Spheginobaccha). RMNH*: HT* M*. [1a]
macropoda Bigot, 1883: 331 (Sphegina). BMNH*: F*. [4: Thompson 1974]
= robusta Brunetti, 1907: 11 (Baccha). ZSI*: A. [4: Thompson 1974]
melancholica Hull, 1937: 174 (Spheginobaccha). USNM: F*. [4: Thompson 1974]
vandoesburgi Thompson, 1974: 273 (Spheginobaccha). BMNH*: HT* M*. [2]
Genus Stipomorpha Hull, 1945 stat. n.
Neotropical
apicula Curran, 1930: 5 (Microdon). AMNH: HT M. [1a] Comb. n.
fraudator Shannon, 1927: 20 (Microdon). BMNH: T M. [1a] Comb. n.
goettei Shannon, 1927: 19 (Microdon). BMNH: T F. [1a] Comb. n.
guianica Curran, 1925: 340 (Microdon). MCZ: T F. [1a] Comb. n.
inarmata Curran, 1925: 5 (Microdon). MCZ: T M. [1a] Comb. n.
lacteipennis Shannon, 1927: 18 (Microdon). BMNH: T M. [1a] Comb. n.
= triangularis Curran, 1940: 6 (Microdon). AMNH: T M. [1a] Syn. n., comb. n.
lanei Curran, 1936: 5 (Microdon). AMNH: HT F. [1a] Comb. n.
litoralis Papavero, 1964: 21 (Ubristes). MZUSP: T M. [1a] Comb. n.
mackiei Curran, 1940: 5 (Microdon). AMNH: HT F. [1a] Comb. n.
micromidas Shannon, 1925: 112 (Microdon). USNM: HT F. [1a] Comb. n.
mixta Curran, 1940: 6 (Microdon). BMNH: T F. [1a] Comb. n.
puerilis Doesburg, 1966: 86 (Ubristes). RMNH*: T F. [1a] Comb. n.
simillima Hull, 1950: 611 (Microdon). BMNH: T M. [1a] Comb. n.
tenuicauda Curran, 1925: 339 (Microdon). CU: T F. [1a] Comb. n.
trigoniformis Shannon, 1927: 19 (Microdon). BMNH: T M. [1a] Comb. n.
wheeleri Mann, 1928: 168 (Microdon). USNM: T M. [1a] Comb. n.
[Descriptions of nine additional species from the Neotropical region are in preparation by the first author.]
Genus Sulcodon Reemer gen. n.
Oriental
sulcatus Hull, 1944: 256 (Microdon). BMNH: HT F. [1a] Comb. n.
Genus Surimyia Reemer, 2008
Neotropical
minutula Doesburg, 1966: 89 (Ceratophya). RMNH: HT* M*. [1a]
rolanderi Reemer, 2008: 180 (Surimyia). RMNH*: HT* M*. [1a]
Genus Thompsodon Reemer gen. n.
Neotropical
conspicillifrons Reemer sp. n. (Thompsodon). INBIO: HT F. [1a] Sp. n.
Genus Ubristes Walker, 1852
Neotropical
flavitibia Walker, 1852: 217 (Ubristes). BMNH: T M. [1a]
= procteri Curran, 1941: 251 (Microdon). AMNH: T M. [1a] Syn. n.
= procedens Curran, 1941: 251 (Microdon). AMNH: T M. [1a] Syn. n.
[Descriptions of two additional species from the Neotropical region are in preparation by the first author.]
Unplaced Microdontinae
Afrotropical
schultzei Simroth, 1907: 796 (Ceratoconcha). T A. Described as mollusc. Only known from larva. Preocc. Kramberger-Gorjanovic, 1889.
Australian / Oceanian
hardyi Ferguson, 1926: 171 (Microdon). Coll. Hardy: T M. [2]
obscurus Wulp, 1898: 421 (Microdon). HNHM (lost)*: T F. [2] Type lost. Van der Wulp (1898) states that the type was a female, but this is doubtful, considering his description of the head.
sharpii Mik, 1900: 148 (Microdon). BMNH: HT* F*. [1a] Replacement name for Microdon pictipennis Sharp, 1899.
= pictipennis Sharp, 1899: 390 (Microdon). BMNH: HT* A. Preocc. Macquart, 1850.
Neotropical
aeolidiformis Wheeler, 1924: 239 (Microdon). USNM: HT* A. [2] Described from larva.
aztecarum Wheeler, 1924: 243 (Nothomicrodon). USNM: T L*. [1a] Described from larva. Uncertain whether it belongs to Syrphidae.
bruesi Hull, 1945: 77 (Microdon). MCZ: HT* F. [1b]
ignobilis Rondani, 1848: 73 (Aphritis). MZUN: ST MF. [2]
longicornis Wiedemann, 1824: 14 (Ceratophya). NMW (lost)*: T F. [2] Type not in NMW (pers. comm. P. Sehnal).
pauper Rondani, 1848: 74 (Aphritis). MZUN: T M. [2]
rubriventris Lynch Arribalzaga, 1891: 128 (Microdon). MACN: T A. [2]
viridis Townsend, 1895: 610 (Microdon). Lost*: HT F*. [4: Thompson 1981b] See Thompson (1981b).
Oriental
apidiformis Brunetti, 1925: 78 (Microdon). ZSI*: M*. [2] Replacement name for Microdon apiformis Brunetti, 1923.
= apiformis Brunetti, 1923: 314 (Microdon). ZSI: HT M. Preocc. De Geer, 1776.
dimidiatus Curran, 1942: 3 (Microdon). BMNH (lost)*: HT M. [2] Type not found in AMNH and BMNH.
laxiceps Curran, 1942: 2 (Microdon). BMNH (lost)*: HT F. [2] Type not found in AMNH and BMNH.
shirakii Nom. n. New replacement name for Microdon tuberculatus Shiraki, 1968. See notes under Genus account of Kryptopyga.
= tuberculatus Shiraki, 1968: 11 (Microdon). NIAS: HT F. [1a] Preocc. de Meijere, 1913.
trigonospilus Bezzi, 1927: 4 (Microdon). MCSN*: HT* F*. [1a]
Taxa previously considered to belong to Microdontinae
Afrotropical
varius Walker, 1849: 540 (Microdon). F*. [4: Thompson 2010] Species of Graptomyza (Syrphidae: Eristalinae).
Neotropical
grandis Lynch Arribalzaga, 1892: 255 (Argentinomyia). MACN*: A. [4] Species of Argentinomyia (Syrphidae: Syrphinae)
testaceipes Lynch Arribalzaga, 1891: 199 (Argentinomyia). MACN*: A. [4] Species of Argentinomyia (Syrphidae: Syrphinae)
Palaearctic
apiarius Fabricius, 1781: 422 (Syrphus). A [4: Thompson et al. 1982] Synonym of Mesembrina mystacea (Linneaus) (Muscidae). Wrongly listed by Peck (1988) as synonym of Microdon mutabilis (Linnaeus).
sophianus Drensky, 1934: 122 (Microdon). Lost?: T F. [4: Doczkal and Schmid 1999] Probably a species of Chrysotoxum Meigen (Syrphidae).
3–5 Afromicrodon madecassa male (holotype): 3 habitus dorsal 4 head frontal 5 head lateral 6 Afromicrodon johannae male (paratype), genitalia lateral 7–9 Archimicrodon (s.s.) simplicicornis male (holotype): 7 habitus dorsal 8 habitus lateral 9 genitalia lateral 10 Archimicrodon (s.s.) malukensis male (holotype), habitus dorsal.
3–5 Afromicrodon madecassa male (holotype): 3 habitus dorsal 4 head frontal 5 head lateral 6 Afromicrodon johannae male (paratype), genitalia lateral 7–9 Archimicrodon (s.s.) simplicicornis male (holotype): 7 habitus dorsal 8 habitus lateral 9 genitalia lateral 10 Archimicrodon (s.s.) malukensis male (holotype), habitus dorsal.
11–15 Archimicrodon (s.s) malukensis: 11 male (holotype), habitus lateral 12 male (holotype), head frontal 13 female (paratype), head frontal 14 male (holotype, wing 15 male (paratype), genitalia lateral 16–17 Archimicrodon (Hovamicrodon) silvester male (holotype): 16 habitus lateral 17 wing 18–19 Archimicrodon (Hovamicrodon): 18 Archimicrodon nubecula female (holotype), scutellum 19 Archimicrodon silvester male (holotype), genitalia lateral.
11–15 Archimicrodon (s.s) malukensis: 11 male (holotype), habitus lateral 12 male (holotype), head frontal 13 female (paratype), head frontal 14 male (holotype, wing 15 male (paratype), genitalia lateral 16–17 Archimicrodon (Hovamicrodon) silvester male (holotype): 16 habitus lateral 17 wing 18–19 Archimicrodon (Hovamicrodon): 18 Archimicrodon nubecula female (holotype), scutellum 19 Archimicrodon silvester male (holotype), genitalia lateral.
20–22 Archimicrodon (s.l.) fenestrellatus male (holotype): 20 habitus dorsal 21 habitus lateral 22 genitalia lateral 23–26 Archimicrodon (s.l.), male genitalia lateral: 23 Archimicrodon brevicornis (syntype) 24 Archimicrodon simplex (South Korea, coll. RMNH) 25 Archimicrodon venosus (holotype Microdon papuanus van Doesburg, jun. syn.) 26 Archimicrodon cf. fergusoni (Australia, coll. CSCA).
20–22 Archimicrodon (s.l.) fenestrellatus male (holotype): 20 habitus dorsal 21 habitus lateral 22 genitalia lateral 23–26 Archimicrodon (s.l.), male genitalia lateral: 23 Archimicrodon brevicornis (syntype) 24 Archimicrodon simplex (South Korea, coll. RMNH) 25 Archimicrodon venosus (holotype Microdon papuanus van Doesburg, jun. syn.) 26 Archimicrodon cf. fergusoni (Australia, coll. CSCA).
27–29 Aristosyrphus primus male: 27 habitus dorsal (Brazil, coll. J.T. Smit) 28 wing (Brazil, coll. J.T. Smit) 29 genitalia lateral (Brazil, coll. SEMC) 30–34 Aristosyrphus (Eurypterosyrphus) males: 30 Aristosyrphus currani male (holotype), habitus lateral 31 Aristosyrphus. spec. (Brazil, coll. ZMAN), head lateral 32 Aristosyrphus spec. #1 (Costa Rica, coll. ZMAN), genitalia ventral 33 Aristosyrphus spec. #2 (Brazil, coll. ZMAN), genitalia lateral 34 Aristosyrphus spec. #1 (Costa Rica, coll. ZMAN), genitalia lateral.
27–29 Aristosyrphus primus male: 27 habitus dorsal (Brazil, coll. J.T. Smit) 28 wing (Brazil, coll. J.T. Smit) 29 genitalia lateral (Brazil, coll. SEMC) 30–34 Aristosyrphus (Eurypterosyrphus) males: 30 Aristosyrphus currani male (holotype), habitus lateral 31 Aristosyrphus. spec. (Brazil, coll. ZMAN), head lateral 32 Aristosyrphus spec. #1 (Costa Rica, coll. ZMAN), genitalia ventral 33 Aristosyrphus spec. #2 (Brazil, coll. ZMAN), genitalia lateral 34 Aristosyrphus spec. #1 (Costa Rica, coll. ZMAN), genitalia lateral.
35–37 Bardistopus papuanum male (holotype): 35 habitus dorsal 36 habitus lateral 37 genitalia lateral 38–41 Carreramyia megacephalus male (Costa Rica, coll. M. Hauser): 38 habitus dorsal 39 head frontal 40 habitus lateral 41 genitalia lateral (Costa Rica, coll. RMNH) 42–43 Ceratophya notata male (holotype): 42 habitus dorsal 43 habitus lateral.
35–37 Bardistopus papuanum male (holotype): 35 habitus dorsal 36 habitus lateral 37 genitalia lateral 38–41 Carreramyia megacephalus male (Costa Rica, coll. M. Hauser): 38 habitus dorsal 39 head frontal 40 habitus lateral 41 genitalia lateral (Costa Rica, coll. RMNH) 42–43 Ceratophya notata male (holotype): 42 habitus dorsal 43 habitus lateral.
44–45 Ceratophya: 44 Ceratophya panamensis female (paratype), abdomen lateral 45 Ceratophya notata male (holotype), genitalia 46–52 Ceratrichomyia males (holotypes): 46 Ceratophya behara, habitus dorsal 47 Ceratophya behara, habitus lateral 48 Ceratophya bullabucca, habitus dorsal 49 Ceratophya bullabucca, habitus lateral 50 Ceratophya bullabucca, head frontal 51 Ceratophya angolensis, habitus dorsal 52 Ceratophya angolensis, habitus lateral.
44–45 Ceratophya: 44 Ceratophya panamensis female (paratype), abdomen lateral 45 Ceratophya notata male (holotype), genitalia 46–52 Ceratrichomyia males (holotypes): 46 Ceratophya behara, habitus dorsal 47 Ceratophya behara, habitus lateral 48 Ceratophya bullabucca, habitus dorsal 49 Ceratophya bullabucca, habitus lateral 50 Ceratophya bullabucca, head frontal 51 Ceratophya angolensis, habitus dorsal 52 Ceratophya angolensis, habitus lateral.
53–55 Ceratrichomyia angolensis male (holotype): 53 head frontal 54 head lateral 55 wing. 56–58 Ceratrichomyia males (holotypes): 56 Ceratrichomyia angolensis 57 Ceratrichomyia behara 58 Ceratrichomyia bullabucca 59–60 Ceriomicrodon petiolatus male (holotype): 59 habitus lateral 60 genitalia lateral.
53–55 Ceratrichomyia angolensis male (holotype): 53 head frontal 54 head lateral 55 wing. 56–58 Ceratrichomyia males (holotypes): 56 Ceratrichomyia angolensis 57 Ceratrichomyia behara 58 Ceratrichomyia bullabucca 59–60 Ceriomicrodon petiolatus male (holotype): 59 habitus lateral 60 genitalia lateral.
61–62 Cervicorniphora alcicornis male (Australia, coll. USNM): 61 habitus lateral 62 genitalia lateral 63–65 Chrysidimyia chrysidimima male (holotype): 63 habitus dorsal 64 habitus lateral 65 genitalia lateral 66–67 Chrysidimyia chrysidimima male (holotype): 66 head frontal 67 head lateral.
61–62 Cervicorniphora alcicornis male (Australia, coll. USNM): 61 habitus lateral 62 genitalia lateral 63–65 Chrysidimyia chrysidimima male (holotype): 63 habitus dorsal 64 habitus lateral 65 genitalia lateral 66–67 Chrysidimyia chrysidimima male (holotype): 66 head frontal 67 head lateral.
68–73 Domodon zodiacus male (holotype): 68 habitus dorsal 69 habitus lateral 70 head frontal 71 head lateral 72 wing 73 genitalia lateral 74–75 Furcantenna nepalensis male (holotype): 74 habitus dorsal 75 habitus lateral.
68–73 Domodon zodiacus male (holotype): 68 habitus dorsal 69 habitus lateral 70 head frontal 71 head lateral 72 wing 73 genitalia lateral 74–75 Furcantenna nepalensis male (holotype): 74 habitus dorsal 75 habitus lateral.
76–80 Furcantenna nepalensis male (holotype): 76 abdomen dorsal 77 head frontal 78 head lateral 79 wing 80 genitalia lateral 81–82 Heliodon doris male (holotype): 81 habitus dorsal 82 habitus lateral 83–84 Heliodon doris female (paratype): 83 habitus dorsal 84 habitus lateral.
76–80 Furcantenna nepalensis male (holotype): 76 abdomen dorsal 77 head frontal 78 head lateral 79 wing 80 genitalia lateral 81–82 Heliodon doris male (holotype): 81 habitus dorsal 82 habitus lateral 83–84 Heliodon doris female (paratype): 83 habitus dorsal 84 habitus lateral.
85–87 Heliodon doris male (holotype): 85 head frontal 86 head lateral 87 wing 88–90 Heliodon elisabethanna female (holotype): 88 habitus dorsal 89 habitus lateral 90 head frontal 91–92 Heliodon tiber male (holotype): 91 habitus dorsal 92 head frontal 93 Heliodon tiber female (paratype), habitus dorsal.
85–87 Heliodon doris male (holotype): 85 head frontal 86 head lateral 87 wing 88–90 Heliodon elisabethanna female (holotype): 88 habitus dorsal 89 habitus lateral 90 head frontal 91–92 Heliodon tiber male (holotype): 91 habitus dorsal 92 head frontal 93 Heliodon tiber female (paratype), habitus dorsal.
94–96 Heliodon, male genitalia: 94 Heliodon doris (holotype) 95 Heliodon tiber (holotype) 96 Heliodon gloriosus (holotype of Microdon aurivesta Hull, jun. syn.) 97–98 Hypselosyrphus amazonicus female (holotype Microdon scutellaris Shannon): 97 habitus dorsal 98 habitus lateral 99–100 Hypselosyrphus trigonus male (holotype): 99 head frontal 100 head lateral.
94–96 Heliodon, male genitalia: 94 Heliodon doris (holotype) 95 Heliodon tiber (holotype) 96 Heliodon gloriosus (holotype of Microdon aurivesta Hull, jun. syn.) 97–98 Hypselosyrphus amazonicus female (holotype Microdon scutellaris Shannon): 97 habitus dorsal 98 habitus lateral 99–100 Hypselosyrphus trigonus male (holotype): 99 head frontal 100 head lateral.
101–102 Hypselosyrphus, male genitalia: 101 Heliodon amazonicus (Peru, coll. RMNH) (cercus missing) 102 Heliodon analis (holotype) 103–106 Indascia gracilis male (holotype): 103 habitus dorsal 104 habitus lateral 105 wing 106 genitalia 107–108 Indascia gigantica male (holotype): 107 habitus dorsal 108 habitus lateral.
101–102 Hypselosyrphus, male genitalia: 101 Heliodon amazonicus (Peru, coll. RMNH) (cercus missing) 102 Heliodon analis (holotype) 103–106 Indascia gracilis male (holotype): 103 habitus dorsal 104 habitus lateral 105 wing 106 genitalia 107–108 Indascia gigantica male (holotype): 107 habitus dorsal 108 habitus lateral.
109–112 Indascia gigantica male (holotype): 109 head frontal 110 head lateral 111 wing 112 genitalia lateral 113–118 Indascia spathulata male (holotype): 113 habitus lateral 114 dorsal 115 head frontal 116 head lateral 117 wing 118 genitalia lateral.
109–112 Indascia gigantica male (holotype): 109 head frontal 110 head lateral 111 wing 112 genitalia lateral 113–118 Indascia spathulata male (holotype): 113 habitus lateral 114 dorsal 115 head frontal 116 head lateral 117 wing 118 genitalia lateral.
119–124 Kryptopyga pendulosa male (holotype): 119 habitus dorsal 120 habitus lateral 121 head frontal 122 head lateral 123 abdomen ventral 124 wing 125 Kryptopyga pendulosa female (Indonesia, Bangka, coll. RMNH) 126–127 Kryptopyga sulawesiana male (holotype): 126 habitus dorsal 127 habitus lateral.
119–124 Kryptopyga pendulosa male (holotype): 119 habitus dorsal 120 habitus lateral 121 head frontal 122 head lateral 123 abdomen ventral 124 wing 125 Kryptopyga pendulosa female (Indonesia, Bangka, coll. RMNH) 126–127 Kryptopyga sulawesiana male (holotype): 126 habitus dorsal 127 habitus lateral.
Figures 128–129. Kryptopyga sulawesiana male (holotype): 128 head frontal 129 wing 130–131 Kryptopyga, male genitalia 130 Kryptopyga pendulosa (holotype) 131 Kryptopyga sulawesiana (holotype) 132–134 Laetodon violens male (Jamaica, coll. RMNH): 132 habitus dorsal 133 habitus lateral 134 head frontal 135 Laetodon laetus male (USA, Georgia, coll. RMNH), genitalia lateral.
Figures 128–129. Kryptopyga sulawesiana male (holotype): 128 head frontal 129 wing 130–131 Kryptopyga, male genitalia 130 Kryptopyga pendulosa (holotype) 131 Kryptopyga sulawesiana (holotype) 132–134 Laetodon violens male (Jamaica, coll. RMNH): 132 habitus dorsal 133 habitus lateral 134 head frontal 135 Laetodon laetus male (USA, Georgia, coll. RMNH), genitalia lateral.
136–138 Masarygus planifrons male (syntype): 136 habitus dorsal 137 habitus lateral 138 head frontal 139 Masarygus planifrons female (syntype), habitus dorsal 140–143 Masarygus palmipalpus male (holotype): 140 habitus dorsal 141 habitus lateral 142 head frontal 143 head lateral 144 antenna.
136–138 Masarygus planifrons male (syntype): 136 habitus dorsal 137 habitus lateral 138 head frontal 139 Masarygus planifrons female (syntype), habitus dorsal 140–143 Masarygus palmipalpus male (holotype): 140 habitus dorsal 141 habitus lateral 142 head frontal 143 head lateral 144 antenna.
145–146 Masarygus palmipalpus male (holotype): 145 wing 146 genitalia lateral 147–149 Masarygus spec. 1 male (Brazil, coll. USNM): 147 habitus dorsal 148 habitus lateral 149 head frontal 150 Masarygus spec. 2 male (Brazil, coll. USNM), habitus lateral 151–153 Menidon falcatus male (Costa Rica, coll. ZMAN): 151 habitus dorsal 152 habitus lateral 153 head frontal.
145–146 Masarygus palmipalpus male (holotype): 145 wing 146 genitalia lateral 147–149 Masarygus spec. 1 male (Brazil, coll. USNM): 147 habitus dorsal 148 habitus lateral 149 head frontal 150 Masarygus spec. 2 male (Brazil, coll. USNM), habitus lateral 151–153 Menidon falcatus male (Costa Rica, coll. ZMAN): 151 habitus dorsal 152 habitus lateral 153 head frontal.
154–155 Menidon falcatus male (Costa Rica, coll. ZMAN): 154 antenna 155 wing 156 Menidon falcatus male (Costa Rica, coll M. Hauser), genitalia lateral 157–162 Mermerizon inbio male (holotype): 157 habitus lateral 158 habitus dorsal 159 head frontal 160 head lateral 161 wing 162 genitalia lateral.
154–155 Menidon falcatus male (Costa Rica, coll. ZMAN): 154 antenna 155 wing 156 Menidon falcatus male (Costa Rica, coll M. Hauser), genitalia lateral 157–162 Mermerizon inbio male (holotype): 157 habitus lateral 158 habitus dorsal 159 head frontal 160 head lateral 161 wing 162 genitalia lateral.
163–166 Metadon achterbergi female (holotype) 163 habitus dorsal 164 habitus lateral 165 head frontal 166 wing 167–168 Metadon, habitus: 167 Metadon bifasciatus (Japan, coll. RMNH) 168 Metadon inermis (holotype) 169–170 Metadon wulpii female (Borneo, coll. RMNH): 169 habitus dorsal 170 habitus lateral.
163–166 Metadon achterbergi female (holotype) 163 habitus dorsal 164 habitus lateral 165 head frontal 166 wing 167–168 Metadon, habitus: 167 Metadon bifasciatus (Japan, coll. RMNH) 168 Metadon inermis (holotype) 169–170 Metadon wulpii female (Borneo, coll. RMNH): 169 habitus dorsal 170 habitus lateral.
Figures 171–175. Metadon, male genitalia lateral: 171 Metadon bicoloratus (holotype) 172 Metadon bifasciatus (China, coll. RMNH) 173 Metadon inermis (holotype) 174 Metadon montis (holotype) 175 Metadon punctulatus (holotype).
Figures 171–175. Metadon, male genitalia lateral: 171 Metadon bicoloratus (holotype) 172 Metadon bifasciatus (China, coll. RMNH) 173 Metadon inermis (holotype) 174 Metadon montis (holotype) 175 Metadon punctulatus (holotype).
176–178 Microdon (Chymophila) instabilis (holotype): 176 habitus dorsal 177 wing 178 genitalia lateral 179–180 Microdon (Chymophila), male genitalia: 179 Microdon lativentris (holotype) 180 Microdon stramineus (Surinam, coll. RMNH).
176–178 Microdon (Chymophila) instabilis (holotype): 176 habitus dorsal 177 wing 178 genitalia lateral 179–180 Microdon (Chymophila), male genitalia: 179 Microdon lativentris (holotype) 180 Microdon stramineus (Surinam, coll. RMNH).
181–182 Microdon (Dimeraspis) marmoratus male (holotype): 181 habitus dorsal 182 habitus lateral 183 Microdon (Dimeraspis) globosus male (USA, Pennsylvania, coll. RMNH), scutellum 184–186 Microdon (Dimeraspis), male genitalia: 184 Microdon globosus (USA, Pennsylvania, coll. RMNH) 185 Microdon abditus (paratype, USA, Queens, coll. RMNH) 186 Microdon fuscipennis (USA, N-Carolina, coll. RMNH).
181–182 Microdon (Dimeraspis) marmoratus male (holotype): 181 habitus dorsal 182 habitus lateral 183 Microdon (Dimeraspis) globosus male (USA, Pennsylvania, coll. RMNH), scutellum 184–186 Microdon (Dimeraspis), male genitalia: 184 Microdon globosus (USA, Pennsylvania, coll. RMNH) 185 Microdon abditus (paratype, USA, Queens, coll. RMNH) 186 Microdon fuscipennis (USA, N-Carolina, coll. RMNH).
187–190 Microdon (Megodon) stuckenbergi male (holotype): 187 habitus dorsal 188 thorax dorsal 189 habitus lateral 190 head frontal 191–193 Microdon (Megodon) stuckenbergi male (holotype): 191 head lateral 192 wing 193 genitalia 194–195 Microdon (Megodon) planitarsus male (holotype): 194 habitus dorsal 195 genitalia.
187–190 Microdon (Megodon) stuckenbergi male (holotype): 187 habitus dorsal 188 thorax dorsal 189 habitus lateral 190 head frontal 191–193 Microdon (Megodon) stuckenbergi male (holotype): 191 head lateral 192 wing 193 genitalia 194–195 Microdon (Megodon) planitarsus male (holotype): 194 habitus dorsal 195 genitalia.
196–199 Microdon (Microdon) hauseri male (holotype): 196 habitus lateral 197 abdomen dorsal 198 hind femur, frontal 199 head frontal 200–203 Microdon (Microdon) mandarinus male (holotype): 200 habitus dorsal 201 head frontal 202 habitus lateral 203 head dorsal. 204–205 Microdon (Microdon) mandarinus female (paratype): 204 habitus dorsal 205 habitus lateral.
196–199 Microdon (Microdon) hauseri male (holotype): 196 habitus lateral 197 abdomen dorsal 198 hind femur, frontal 199 head frontal 200–203 Microdon (Microdon) mandarinus male (holotype): 200 habitus dorsal 201 head frontal 202 habitus lateral 203 head dorsal. 204–205 Microdon (Microdon) mandarinus female (paratype): 204 habitus dorsal 205 habitus lateral.
206 Microdon (Microdon) mandarinus male (holotype), wing 207–210 Microdon (Microdon) yunnanensis male (holotype) 207 habitus lateral 208 habitus dorsal 209 head frontal 210 wing 211–212 Microdon (Microdon), male genitalia: 211 Microdon mutabilis (Belarus, coll. M. Reemer) 212 Microdon hauseri (holotype).
206 Microdon (Microdon) mandarinus male (holotype), wing 207–210 Microdon (Microdon) yunnanensis male (holotype) 207 habitus lateral 208 habitus dorsal 209 head frontal 210 wing 211–212 Microdon (Microdon), male genitalia: 211 Microdon mutabilis (Belarus, coll. M. Reemer) 212 Microdon hauseri (holotype).
213–214 Microdon (Microdon), male genitalia: 213 Microdon mandarinus (holotype) 214 Microdon yunnanensis (holotype) 215–220 Microdon (Myiacerapis) villosus male (holotype): 215 habitus dorsal 216 habitus lateral 217 head frontal 218 head dorsal 219 wing 220 genitalia lateral.
213–214 Microdon (Microdon), male genitalia: 213 Microdon mandarinus (holotype) 214 Microdon yunnanensis (holotype) 215–220 Microdon (Myiacerapis) villosus male (holotype): 215 habitus dorsal 216 habitus lateral 217 head frontal 218 head dorsal 219 wing 220 genitalia lateral.
221–222 Microdon (Syrphipogon) fucatissimus male (holotype): 221 habitus lateral 222 genitalia lateral 223–228 Microdon s.l. males, habitus: 223 craigheadi-group: Microdon craigheadi (USA, S-Carolina, coll. BMNH) 224 erythros-group: Microdon luteiventris (Kenya, coll. RMNH) 225 mirabilis-group: Microdon bertonii (Brazil, coll. RMNH) 226 tarsalis-group: Microdon tarsalis (holotype) 227 Microdon tarsalis 228 virgo-group: Microdon rufiventris (Surinam, coll. RMNH).
221–222 Microdon (Syrphipogon) fucatissimus male (holotype): 221 habitus lateral 222 genitalia lateral 223–228 Microdon s.l. males, habitus: 223 craigheadi-group: Microdon craigheadi (USA, S-Carolina, coll. BMNH) 224 erythros-group: Microdon luteiventris (Kenya, coll. RMNH) 225 mirabilis-group: Microdon bertonii (Brazil, coll. RMNH) 226 tarsalis-group: Microdon tarsalis (holotype) 227 Microdon tarsalis 228 virgo-group: Microdon rufiventris (Surinam, coll. RMNH).
229 Microdon (virgo-group) rufiventris (Surinam, coll. RMNH), head lateral 230–231 Microdon (s.l.), male, habitus: 230 Microdon pictipennis (Australia, coll. MNHN) 231 Microdon tsara (holotype) 232–236 Microdon (s.l.), male genitalia: 232 Microdon craigheadi (USA, Georgia, coll. RMNH) 233 Microdon erythros (Congo, coll. RMNH) 234 Microdon bertonii (Brazil, coll. RMNH) 235 Microdon tarsalis (holotype) 236 Microdon rufiventris (Surinam, coll. RMNH).
229 Microdon (virgo-group) rufiventris (Surinam, coll. RMNH), head lateral 230–231 Microdon (s.l.), male, habitus: 230 Microdon pictipennis (Australia, coll. MNHN) 231 Microdon tsara (holotype) 232–236 Microdon (s.l.), male genitalia: 232 Microdon craigheadi (USA, Georgia, coll. RMNH) 233 Microdon erythros (Congo, coll. RMNH) 234 Microdon bertonii (Brazil, coll. RMNH) 235 Microdon tarsalis (holotype) 236 Microdon rufiventris (Surinam, coll. RMNH).
237–240 Microdon (s.l.), male genitalia: 237 Microdon carbonarius (holotype) 238 Microdon pictipennis (Australia, coll. W. van Steenis) 239 Microdon tsara (holotype) 240 Microdon waterhousei (Australia, coll. M. Hauser) 241–242 Mixogaster breviventris male (USA, coll. RMNH): 241 habitus dorsal 242 wing 242–244 Mixogaster thecla male (Brazil, coll. J.T. Smit): 243 habitus dorsal 244 wing.
237–240 Microdon (s.l.), male genitalia: 237 Microdon carbonarius (holotype) 238 Microdon pictipennis (Australia, coll. W. van Steenis) 239 Microdon tsara (holotype) 240 Microdon waterhousei (Australia, coll. M. Hauser) 241–242 Mixogaster breviventris male (USA, coll. RMNH): 241 habitus dorsal 242 wing 242–244 Mixogaster thecla male (Brazil, coll. J.T. Smit): 243 habitus dorsal 244 wing.
245–246 Mixogaster breviventris male (USA, coll. RMNH), genitalia: 245 lateral 246 ventral. Figure 247. Mixogaster thecla male (Brazil, coll. J.T. Smit), genitalia lateral 248 Mixogaster spec. nov. male (Colombia, coll. RMNH), aedeagus ventral 249–250 Oligeriops dimorphon female (Australia, coll. BMNH): 249 habitus dorsal 250 habitus lateral.
245–246 Mixogaster breviventris male (USA, coll. RMNH), genitalia: 245 lateral 246 ventral. Figure 247. Mixogaster thecla male (Brazil, coll. J.T. Smit), genitalia lateral 248 Mixogaster spec. nov. male (Colombia, coll. RMNH), aedeagus ventral 249–250 Oligeriops dimorphon female (Australia, coll. BMNH): 249 habitus dorsal 250 habitus lateral.
251–252 Oligeriops iridomyrmex female (syntype): 251 head frontal 252 head lateral 253 Oligeriops dimorphon male (Australia, coll. USNM), genitalia lateral 254–255 Omegasyrphus pallipennis male (USA, California, coll. RMNH) 254 habitus dorsal 255 habitus lateral 256 Omegasyrphus coarctatus male (USA, Virginia, coll. RMNH), genitalia lateral. 257–258 Paragodon paragoides female (Costa Rica, coll. RMNH): 257 habitus lateral 258 wing.
251–252 Oligeriops iridomyrmex female (syntype): 251 head frontal 252 head lateral 253 Oligeriops dimorphon male (Australia, coll. USNM), genitalia lateral 254–255 Omegasyrphus pallipennis male (USA, California, coll. RMNH) 254 habitus dorsal 255 habitus lateral 256 Omegasyrphus coarctatus male (USA, Virginia, coll. RMNH), genitalia lateral. 257–258 Paragodon paragoides female (Costa Rica, coll. RMNH): 257 habitus lateral 258 wing.
259–260 Paragodon paragoides female (Costa Rica, coll. RMNH) 259 head frontal 260 head lateral 261 Paragodon paragoides male (Panama, coll. SEMC), genitalia lateral. 262 Paramicrodon flukei male (holotype), habitus dorsal. Photo: American Museum of Natural History 263–266 Paramicrodon toxopei male (holotype): 263 habitus dorsal 264 habitus lateral 265 head frontal 266 head lateral 267–268 Paramicrodon, male genitalia: 267 Paramicrodon cf. flukei (Peru, coll. RMNH) 268 Microdon toxopei (holotype).
259–260 Paragodon paragoides female (Costa Rica, coll. RMNH) 259 head frontal 260 head lateral 261 Paragodon paragoides male (Panama, coll. SEMC), genitalia lateral. 262 Paramicrodon flukei male (holotype), habitus dorsal. Photo: American Museum of Natural History 263–266 Paramicrodon toxopei male (holotype): 263 habitus dorsal 264 habitus lateral 265 head frontal 266 head lateral 267–268 Paramicrodon, male genitalia: 267 Paramicrodon cf. flukei (Peru, coll. RMNH) 268 Microdon toxopei (holotype).
269–270 Paramixogaster acantholepidis male (holotype): 269 habitus dorsal 270 habitus lateral 271 Paramixogaster luxor male (holotype), habitus dorsal 272–275 Paramixogaster piptotus female (holotype): 272 habitus dorsal 273 head frontal 274 thorax dorsal 275 habitus lateral 276–277 Paramixogaster, habitus dorsal: 276 Paramixogaster omeanus male (holotype) 277 Paramixogaster vespiformis female (syntype).
269–270 Paramixogaster acantholepidis male (holotype): 269 habitus dorsal 270 habitus lateral 271 Paramixogaster luxor male (holotype), habitus dorsal 272–275 Paramixogaster piptotus female (holotype): 272 habitus dorsal 273 head frontal 274 thorax dorsal 275 habitus lateral 276–277 Paramixogaster, habitus dorsal: 276 Paramixogaster omeanus male (holotype) 277 Paramixogaster vespiformis female (syntype).
278–279 Paramixogaster vespiformis female (syntype): 278 habitus lateral 279 head frontal 280–284 Paramixogaster, male genitalia: 280 Paramixogaster acantholepidis (holotype) 281 Paramixogaster illucens (holotype) 282 Paramixogaster luxor (holotype) 283 Paramixogaster omeanus (Australia, coll. USNM) 284 Paramixogaster cf. vespiformis (Vietnam, coll. RMNH).
278–279 Paramixogaster vespiformis female (syntype): 278 habitus lateral 279 head frontal 280–284 Paramixogaster, male genitalia: 280 Paramixogaster acantholepidis (holotype) 281 Paramixogaster illucens (holotype) 282 Paramixogaster luxor (holotype) 283 Paramixogaster omeanus (Australia, coll. USNM) 284 Paramixogaster cf. vespiformis (Vietnam, coll. RMNH).
285–289 Parocyptamus sonamii male (syntype, except 290 Thailand, coll. RMNH): 285 habitus dorsal 286 habitus lateral 287 tergite 2 dorsal 288 head frontal 289 wing 290 Parocyptamus stenogaster male (holotype), genitalia lateral 291–292 Peradon bidens male (Surinam, coll. RMNH): 291 face frontal 292 head lateral.
285–289 Parocyptamus sonamii male (syntype, except 290 Thailand, coll. RMNH): 285 habitus dorsal 286 habitus lateral 287 tergite 2 dorsal 288 head frontal 289 wing 290 Parocyptamus stenogaster male (holotype), genitalia lateral 291–292 Peradon bidens male (Surinam, coll. RMNH): 291 face frontal 292 head lateral.
293 Peradon bidens (Surinam, coll. RMNH), habitus dorsal 294 Peradon flavofascium female (Surinam, coll. RMNH), habitus dorsal 295 Peradon trivittatum male (Surinam, coll. RMNH), habitus dorsal 296–298 Peradon, male genitalia lateral: 296 Peradon bidens (Surinam, coll. RMNH) 296 Peradon flavofascium (holotype) 298 Peradon trivittatum (Surinam, coll. RMNH).
293 Peradon bidens (Surinam, coll. RMNH), habitus dorsal 294 Peradon flavofascium female (Surinam, coll. RMNH), habitus dorsal 295 Peradon trivittatum male (Surinam, coll. RMNH), habitus dorsal 296–298 Peradon, male genitalia lateral: 296 Peradon bidens (Surinam, coll. RMNH) 296 Peradon flavofascium (holotype) 298 Peradon trivittatum (Surinam, coll. RMNH).
299–304 Piruwa phaecada male (holotype): 299 habitus dorsal 300 habitus lateral 301 head frontal 302 head lateral 303 wing 304 genitalia lateral 305–306 Piruwa phaecada female (paratype) 305 habitus dorsal 306 habitus lateral 307 Pseudomicrodon polistoides female (holotype), habitus dorsal.
299–304 Piruwa phaecada male (holotype): 299 habitus dorsal 300 habitus lateral 301 head frontal 302 head lateral 303 wing 304 genitalia lateral 305–306 Piruwa phaecada female (paratype) 305 habitus dorsal 306 habitus lateral 307 Pseudomicrodon polistoides female (holotype), habitus dorsal.
308–311 Pseudomicrodon polistoides female (holotype) 308 habitus lateral 309 head frontal 310 head lateral 311 thorax dorsal 312–316 Pseudomicrodon smiti male (holotype): 312 habitus dorsal 313 habitus lateral 314 head frontal 315 head lateral 316 wing.
308–311 Pseudomicrodon polistoides female (holotype) 308 habitus lateral 309 head frontal 310 head lateral 311 thorax dorsal 312–316 Pseudomicrodon smiti male (holotype): 312 habitus dorsal 313 habitus lateral 314 head frontal 315 head lateral 316 wing.
317–319 Pseudomicrodon biluminiferus male (holotype): 317 habitus dorsal 318 habitus lateral 319 genitalia lateral 320 Pseudomicrodon smiti male (holotype), genitalia lateral 321–324 Ptilobactrum neavei male (holotype): 321 habitus dorsal 322 habitus lateral 323 head frontal 324 wing.
317–319 Pseudomicrodon biluminiferus male (holotype): 317 habitus dorsal 318 habitus lateral 319 genitalia lateral 320 Pseudomicrodon smiti male (holotype), genitalia lateral 321–324 Ptilobactrum neavei male (holotype): 321 habitus dorsal 322 habitus lateral 323 head frontal 324 wing.
325–326 Ptilobactrum neavei male (holotype): 325 antennae 326 genitalia lateral 327–328 Rhoga sepulchrasilva male (Brazil, coll. USNM): 327 head frontal 328 head lateral 329–330 Rhoga mellea male (holotype): 329 habitus lateral 330 habitus dorsal 331 Rhoga sepulchrasilva male (Brazil, coll. USNM), genitalia lateral.
325–326 Ptilobactrum neavei male (holotype): 325 antennae 326 genitalia lateral 327–328 Rhoga sepulchrasilva male (Brazil, coll. USNM): 327 head frontal 328 head lateral 329–330 Rhoga mellea male (holotype): 329 habitus lateral 330 habitus dorsal 331 Rhoga sepulchrasilva male (Brazil, coll. USNM), genitalia lateral.
332–336 Rhopalosyrphus ecuadoriensis male (holotype): 332 habitus dorsal 333 habitus lateral 334 head lateral 335 head frontal 336 wing 337–341 Rhopalosyrphus robustus female (holotype) 337 habitus dorsal 338 habitus lateral 339 head lateral 340 head frontal 341 wing.
332–336 Rhopalosyrphus ecuadoriensis male (holotype): 332 habitus dorsal 333 habitus lateral 334 head lateral 335 head frontal 336 wing 337–341 Rhopalosyrphus robustus female (holotype) 337 habitus dorsal 338 habitus lateral 339 head lateral 340 head frontal 341 wing.
342–345 Rhopalosyrphus (s.l.) abnormoides male (holotype): 342 habitus dorsal 343 habitus lateral 344 head lateral 345 head frontal 346 Rhopalosyrphus (s.l.) cerioides male (holotype), habitus dorsal 347–350 Rhopalosyrphus (s.l.) oreokawensis male (holotype): 347 habitus dorsal 348 habitus lateral 349 wing 350 head frontal.
342–345 Rhopalosyrphus (s.l.) abnormoides male (holotype): 342 habitus dorsal 343 habitus lateral 344 head lateral 345 head frontal 346 Rhopalosyrphus (s.l.) cerioides male (holotype), habitus dorsal 347–350 Rhopalosyrphus (s.l.) oreokawensis male (holotype): 347 habitus dorsal 348 habitus lateral 349 wing 350 head frontal.
351 Rhopalosyrphus (s.l.) oreokawensis male (holotype), head lateral 352–355 Rhopalosyrphus, male genitalia: 352 Rhopalosyrphus ecuadoriensis 353 Rhopalosyrphus abnormoides 354 Rhopalosyrphus cerioides 355 Rhopalosyrphus oreokawensis.
351 Rhopalosyrphus (s.l.) oreokawensis male (holotype), head lateral 352–355 Rhopalosyrphus, male genitalia: 352 Rhopalosyrphus ecuadoriensis 353 Rhopalosyrphus abnormoides 354 Rhopalosyrphus cerioides 355 Rhopalosyrphus oreokawensis.
356–360 Schizoceratomyia barretoi male (Surinam, coll. RMNH): 356 habitus dorsal 357 habitus lateral 358 antenna 359 head frontal 360 head lateral 361 Schizoceratomyia barretoi female (Brazil, coll. RMNH), head lateral 362 Schizoceratomyia barretoi male (Surinam, coll. RMNH), genitalia lateral 363 Schizoceratomyia flavipes male (Surinam, coll. RMNH), genitalia lateral.
356–360 Schizoceratomyia barretoi male (Surinam, coll. RMNH): 356 habitus dorsal 357 habitus lateral 358 antenna 359 head frontal 360 head lateral 361 Schizoceratomyia barretoi female (Brazil, coll. RMNH), head lateral 362 Schizoceratomyia barretoi male (Surinam, coll. RMNH), genitalia lateral 363 Schizoceratomyia flavipes male (Surinam, coll. RMNH), genitalia lateral.
364–369 Serichlamys rufipes male (holotype): 364 habitus dorsal 365 habitus lateral 366 head frontal 367 head lateral 368 wing 369 genitalia lateral 370–371 Serichlamys scutifer (USA, Texas, coll. RMNH): 370 habitus lateral 371 genitalia lateral.
364–369 Serichlamys rufipes male (holotype): 364 habitus dorsal 365 habitus lateral 366 head frontal 367 head lateral 368 wing 369 genitalia lateral 370–371 Serichlamys scutifer (USA, Texas, coll. RMNH): 370 habitus lateral 371 genitalia lateral.
372–373 Serichlamys mitis male (Brazil, coll. RMNH): 372 habitus dorsal 373 habitus lateral 374–375 Serichlamys spec.: 374 male, scutellum (Brazil, coll. RMNH) 375 female, habitus (Ecuador, coll. RBIN) 376 Serichlamys mitis male (Brazil, coll. RMNH), genitalia lateral 377–378 Spheginobaccha macropoda male (Vietnam, coll. RMNH): 377 habitus dorsal 378 habitus lateral
372–373 Serichlamys mitis male (Brazil, coll. RMNH): 372 habitus dorsal 373 habitus lateral 374–375 Serichlamys spec.: 374 male, scutellum (Brazil, coll. RMNH) 375 female, habitus (Ecuador, coll. RBIN) 376 Serichlamys mitis male (Brazil, coll. RMNH), genitalia lateral 377–378 Spheginobaccha macropoda male (Vietnam, coll. RMNH): 377 habitus dorsal 378 habitus lateral
379–381 Spheginobaccha macropoda male (Vietnam, coll. RMNH): 379 head lateral 380 head frontal 381 wing 382–384 Spheginobaccha guttula male (holotype): 382 habitus dorsal 383 head frontal 384 head lateral 385–387 Spheginobaccha, male genitalia: 385 Spheginobaccha macropoda (Vietnam, coll. RMNH), lateral 386 Spheginobaccha guttula (holotype), hypandrium, including aedeagus, lateral 387 Spheginobaccha guttula (holotype), ventral.
379–381 Spheginobaccha macropoda male (Vietnam, coll. RMNH): 379 head lateral 380 head frontal 381 wing 382–384 Spheginobaccha guttula male (holotype): 382 habitus dorsal 383 head frontal 384 head lateral 385–387 Spheginobaccha, male genitalia: 385 Spheginobaccha macropoda (Vietnam, coll. RMNH), lateral 386 Spheginobaccha guttula (holotype), hypandrium, including aedeagus, lateral 387 Spheginobaccha guttula (holotype), ventral.
388–391 Stipomorpha tenuicauda female (holotype) 388 habitus dorsal 389 habitus lateral 390 head frontal 391 head lateral 392 Stipomorpha lacteipennis male (holotype), habitus dorsal 393–394 Stipomorpha goettei male (Surinam, coll. RMNH), base of abdomen: 393 lateral 394 ventral 395–396 Stipomorpha, male genitalia: 395 Spheginobaccha tenuicauda (Bolivia, coll. M. Hauser) 396 Spheginobaccha lacteipennis (Surinam, coll. RMNH).
388–391 Stipomorpha tenuicauda female (holotype) 388 habitus dorsal 389 habitus lateral 390 head frontal 391 head lateral 392 Stipomorpha lacteipennis male (holotype), habitus dorsal 393–394 Stipomorpha goettei male (Surinam, coll. RMNH), base of abdomen: 393 lateral 394 ventral 395–396 Stipomorpha, male genitalia: 395 Spheginobaccha tenuicauda (Bolivia, coll. M. Hauser) 396 Spheginobaccha lacteipennis (Surinam, coll. RMNH).
397–399 Sulcodon sulcatus female (Indonesia, Java, coll. RMNH): 397 habitus dorsal 398 habitus lateral 399 genitalia lateral 400–404 Surimyia rolanderi female (Surinam, coll. RMNH): 400 habitus lateral 401 habitus dorsal 402 head frontal 403 head lateral 404 wing 405 Surimyia rolanderi male (holotype), genitalia lateral.
397–399 Sulcodon sulcatus female (Indonesia, Java, coll. RMNH): 397 habitus dorsal 398 habitus lateral 399 genitalia lateral 400–404 Surimyia rolanderi female (Surinam, coll. RMNH): 400 habitus lateral 401 habitus dorsal 402 head frontal 403 head lateral 404 wing 405 Surimyia rolanderi male (holotype), genitalia lateral.
406–411 Thompsodon conspicillifrons female (holotype): 406 habitus dorsal 407 thorax lateral 408 habitus lateral 409 thorax dorsal 410 head frontal 411 head dorsal 412–414 Thompsodon conspicillifrons female (holotype): 412 head lateral 413 frons lateral 414 frons frontal.
406–411 Thompsodon conspicillifrons female (holotype): 406 habitus dorsal 407 thorax lateral 408 habitus lateral 409 thorax dorsal 410 head frontal 411 head dorsal 412–414 Thompsodon conspicillifrons female (holotype): 412 head lateral 413 frons lateral 414 frons frontal.
415–416 Thompsodon conspicillifrons female (holotype): 415 wing 416 abdomen dorsal 417–420 Ubristes flavitibia male (holotype) 417 habitus dorsal 418 habitus lateral 419 head frontal 420 genitalia lateral 421Ubristes spec. female (Brazil, coll. USNM), tergite 2 dorsal.
415–416 Thompsodon conspicillifrons female (holotype): 415 wing 416 abdomen dorsal 417–420 Ubristes flavitibia male (holotype) 417 habitus dorsal 418 habitus lateral 419 head frontal 420 genitalia lateral 421Ubristes spec. female (Brazil, coll. USNM), tergite 2 dorsal.
422–426 Undescribed genus #1 species AUS-01 Thompson in prep., male (Australia, coll. USNM): 422 habitus dorsal 423 habitus lateral 424 head frontal 425 head lateral 426 genitalia lateral 427–429 Undescribed genus #2 species MCR-2 Thompson in prep., male (Costa Rica, coll. RMNH): 427 habitus dorsal 428 wing 429 habitus lateral.
422–426 Undescribed genus #1 species AUS-01 Thompson in prep., male (Australia, coll. USNM): 422 habitus dorsal 423 habitus lateral 424 head frontal 425 head lateral 426 genitalia lateral 427–429 Undescribed genus #2 species MCR-2 Thompson in prep., male (Costa Rica, coll. RMNH): 427 habitus dorsal 428 wing 429 habitus lateral.
430–432 Undescribed genus #2 species MCR-2 male (Costa Rica, coll. RMNH): 430 head frontal 431 genitalia lateral 432 head lateral 433–434 Microdon sharpii female (New Guinea, coll. BMNH, compared with holotype) 433 habitus dorsal 434 habitus lateral 435 Wing veins in Microdontinae (wing base on left side). The dashed line indicates an (imaginary) apical extrapolation of vein RS. In Microdontinae, vein R2+3 is strongly curved basally, resulting in angle A always being larger than angle B.
430–432 Undescribed genus #2 species MCR-2 male (Costa Rica, coll. RMNH): 430 head frontal 431 genitalia lateral 432 head lateral 433–434 Microdon sharpii female (New Guinea, coll. BMNH, compared with holotype) 433 habitus dorsal 434 habitus lateral 435 Wing veins in Microdontinae (wing base on left side). The dashed line indicates an (imaginary) apical extrapolation of vein RS. In Microdontinae, vein R2+3 is strongly curved basally, resulting in angle A always being larger than angle B.