(C) 2013 Jafari R. Kideghesho. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Kideghesho JR, Rija AA, Mwamende KA, Selemani IS (2013) Emerging issues and challenges in conservation of biodiversity in the rangelands of Tanzania. Nature Conservation 6: 1–29. doi: 10.3897/natureconservation.6.5407
Tanzania rangelands are a stronghold for biodiversity harbouring a variety of animal and plant species of economic, ecological and socio-cultural importance. Efforts to protect these resources against destruction and loss have involved, among other things, setting aside some tracks of land as protected areas in the form of national parks, nature reserves, game reserves, game controlled and wildlife management areas. However, these areas and adjacent lands have long been subjected to a number of emerging issues and challenges, which complicate their management, thus putting the resources at risk of over exploitation and extinction. These issues and challenges include, among other things, government policies, failure of conservation (as a form of land use) to compete effectively with alternative land uses, habitat degradation and blockage of wildlife corridors, overexploitation and illegal resource extraction, wildfires, human population growth, poverty, HIV/AIDS pandemic and human-wildlife conflicts. In this paper, we review the emerging issues and challenges in biodiversity conservation by drawing experience from different parts of Tanzania. The paper is based on the premise that, understanding of the issues and challenges underpinning the rangelands is a crucial step towards setting up of plausible objectives, strategies and plans that will improve and lead to effective management of these areas. We conclude by recommending some proactive measures that may enhance the sustainability of the rangeland resources for the benefit of the current and future generations.
Climate change, civil war, habitat degradation, invasive species, illegal hunting, wildfires
Rangelands are characterized by low and erratic precipitation, shallow soils, rough topography and extreme temperatures (Holecheck et al. 2003). These characteristics have rendered most of the rangelands unsuitable for rain-fed agriculture and have therefore led to the notion that rangelands are marginal or wastelands. Rangelands represent 24% of the world’s land area and act as irreplaceable source of livelihood for the poor, supporting about 200 million households and 50% of world’s livestock population (Batelo 2011). However, the notion that rangelands are wastelands seems to be defeated given the number of conflicts among multiple actors who seek to meet their diverse interests in rangelands. Essentially, competition for rangeland resources among different actors is a function of the benefits and values found in these areas.
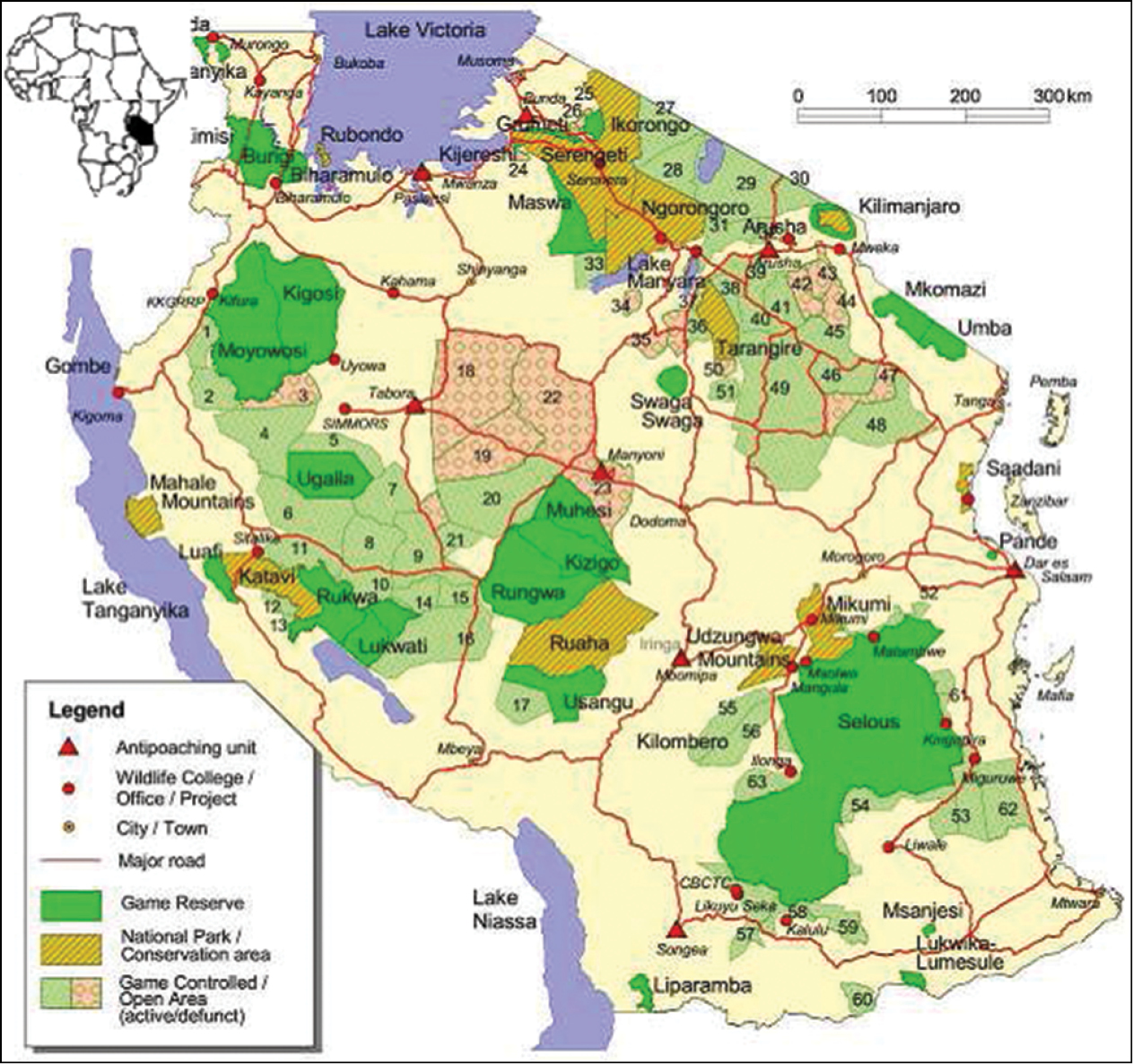
Rangelands are critical areas for biodiversity in terms of genetic material, species and habitats. The diverse nature of habitats found in rangelands is a function of many species of cultural, economic and ecological importance. Tanzania rangelands cover more than 74% of the country land area extending into Dodoma, Mwanza, Kagera, Shinyanga, Arusha, Kilimanjaro, Singida, Tabora and some parts of Iringa, Lindi, Mtwara, Mbeya and Katavi regions (Fig. 1). They are home to the wildlife species considered to be charismatic, umbrella and keystone, endemic and rare. In the sub-Saharan region, Tanzania followed by South Africa surpasses all other 11-member countries of the Southern Africa Development Co-operation (SADC) for having many vertebrates and high endemism (Cumming 1999). Tanzania ranks second highest in terms of the number of vertebrates and plants (Table 1) in the Afro-tropical realm (i.e. all the countries South of the Sahara Desert, including Madagascar). The country possesses about 74% of all plants found in East Africa (Cumming 1999).
Map of Tanzania showing distribution of different protected areas in the rangelands (Map adapted from Baldus and Cauldwell 2004).
Map of Tanzania showing distribution of different protected areas in the rangelands (Map adapted from Baldus and Cauldwell 2004).
The known number of vertebrate animals and plant species in Tanzania (including endemic and threatened species).
| Taxonomic group | Known number | Endemic species | Threatened species | Rank in the Afro-tropical Realm (2) | Number of species per 10 000 Km2 |
|---|---|---|---|---|---|
| Mammals | 316 | 15 | 43 | 4 | 70 |
| Birds | 1, 016 | 24 | 33 | 2 | 184 |
| Reptiles | 289 | 61 | 5 | 3 | 64 |
| Amphibians | 133 | 49 | 0 | 3 | 30 |
| Freshwater fish | – | – | 19 | – | – |
| Higher plants | 10, 008 (Flowering 10, 000) | 1, 122 | 336 | 3 | 2, 231 |
| Taxonomic group | Known number | Endemic species | Threatened species*** | **Rank in the Afro-tropical Realm (2) | Number of species per 10 000 Km2 |
|---|---|---|---|---|---|
| Mammals | 316 | 15 | 43 | 4 | 70 |
| Birds | 1, 016* | 24 | 33 | 2 | 184 |
| Reptiles | 289 | 61 | 5 | 3 | 64 |
| Amphibians | 133 | 49 | 0 | 3 | 30 |
| Freshwater fish | – | – | 19 | – | – |
| Higher plants | 10, 008 (Flowering 10, 000) | 1, 122 | 336 | 3 | 2, 231 |
Source: *WRI (2001);
Source: *WRI (2001);
**Cumming (1999);
**Cumming (1999);
***IUCN (2002)
***IUCN (2002)
Because of their ecological values and high wildlife concentration, most of the wildlife protected areas in Tanzania are situated in the rangelands. About 40% of Tanzania’s land surface is under one or the other form of protection. The major categories of protected areas include national parks, game reserves, game controlled areas, Ngorongoro Conservation Area and Wildlife Management Areas. Despite commitment and political will, the conservation and management of biodiversity in Tanzania have never been an easy task. There are numerous challenges and issues constraining the conservation work. The aim of this paper is to uncover these issues. We draw examples from different rangelands of Tanzania, where most of the biodiversity resources are found. Our motivation behind this paper is based on the premise that understanding of the issues and challenges underpinning the rangeland ecosystems is a crucial step towards setting up of plausible objectives, strategies and plans that will improve and lead to effective management and conservation of these areas. The paper provides some options for action on what should be done to address the existing challenges.
Loss of biodiversity is a growing trend in virtually all Earth ecosystems. The Millennium Ecosystem Assessment document shows that losses of biodiversity and the related changes in the environment have been more rapid in the past 50 years than ever before in human history (MEA 2005). Many animal and plant populations have declined in numbers, geographical distribution, or both. Species extinction is a natural part of Earth’s history. However, the current losses are the outcomes of human actions. Human activities have increased the extinction rate by at least 100 times more than the natural rate (MEA 2005). Rangelands, like other ecosystems, are vulnerable to loss of biodiversity through five major threats: habitat destruction, overexploitation of species, introduction of exotic species, pollution and global warming. In this section we present the issues and challenges, which have emerged as major drivers or agents in exacerbating these threats in the rangelands of Tanzania.
The establishment of protected areas is construed to be the most feasible strategy of maintaining biodiversity (Chape et al. 2008). Most of the protected areas in sub-Saharan Africa are situated in the rangelands. However, given the multiple uses of the rangelands, decisions to allocate lands for conservation have often faced resistance. This type of land use is perceived as an infringement of the rights of other stakeholders. This is the case when the conservation process involves evicting people from these areas and/or denying them access to the resources critical for their livelihoods (Benjamines et al. 2009). Essentially, for decades, the conventional conservation policies seem to have been accorded higher priority to wildlife than humans. This is illustrated by the following reactions from a number of personalities who wanted the Maasai pastoralists be evicted from Serengeti National Park in the late 1950s:
- “The interests of fauna and flora must come first, those of man and belongings being of secondary importance” – the then Serengeti Park Manager (Neumann 1992: 90).
- “Retaining the Maasai in the park would diminish the value of the area for wildlife and, therefore, risk the interests of the white tourists” – Lee Talbot, an ecologist who led the delegation that was sent to Serengeti by the American Committee for International Wild Life Protection (ACIWLP) to investigate the ecological impact of having Maasai within the park (Bonner 1993).
- The Maasai had no legal right to remain in Serengeti and, if any, should not be greater than the best interests of the rest of the people of the world – Luis Leakey, a paleontologist (Bonner 1993).
- Grzimek used a pen to fight war against Maasai. The popular books and documentaries like ‘No room for animals’ (Grzimek 1956) and ‘Serengeti shall not die’ (Grzimek and Grzimek 1960) depicted apparent bias in favour of the wildlife.
The eviction of the Maasai in order to provide room for wildlife conservation has taken place in almost all rangelands of Tanzania, justified by expansion of national parks and creation of game reserves. For example, Mkomazi Game Reserve [initially, since 1951, the Maasai pastoralists were allowed to live in the reserve but they were evicted in 1986 (Tenga 2000)]; the Mkungunero Game Reserve (1996) in the South of Tarangire National Park; Ikorongo-Grumeti (1994) and Kijereshi Game Reserves in western Serengeti (in 2001); Usangu Game Reserve, which was later annexed to Ruaha National Park. Also, Saadani (formerly, a game reserve) was declared by the government in 2000 to be a national park whereby its boundaries were expanded into the village land. This action created tension between the park managers and local communities whose areas have been taken on the premise that they would be compensated. Importantly, the eviction occurred within the past two decades despite the changed focus of policy aiming at involving local communities in conservation. The expansion of the national park boundaries has further been justified on the basis of redefining the national park borders that have been encroached by the local communities. For example, between 2004 and 2007 Tarangire National Park borders were redefined at the eastern side and extended southward which led to the demolishing of human abodes (almost more than 200 households) and farms. The villages mostly affected by expansion of the park boarders include Gijedabong, Mamire, Mwinkatsi and Endamalamboda. All the identified villages are located in the south-western part of the park (Rija pers. obs. 2006). This has led to an increased hostility between the villagers and park authorities resulting in a lawsuit filed by the villagers over discontentment of the eviction and land ‘grabbing’ by the Tanzania National Park (TANAPA) (Davis 2011). A similar scenario has been observed at the Arusha National Park following an attempt to annex the forest patch adjacent to it. The eviction has overtime worsened the conflicts between these parks and surrounding local communities. Hence, the effort has led to an increased poaching from these protected areas.
Under this scenario where the eviction and prohibitive policies symbolize the conservation process, resentments from local communities and, therefore, conflicts have become the salient features in virtually all rangelands of Tanzania (Goldman 2011, Kaswamila 2010). For example, the Maasai in eastern Serengeti resented the proposed park boundaries through violence and sabotage/vandalism. They resisted the government conservation by spearing the rhinos, setting fires with malicious intent and terrorising civil servants (Neumann 1992). In Western Serengeti, the Ikoma hunters deliberately disobeyed the colonial conservation laws and vowed to kill the wildlife rangers by poisoned arrows particularly when they attempted to stop them from hunting (Neumann 1998). The expansion of Serengeti National Park in the 1960s, which took Kurya’s grazing, arable and hunting land, culminated into resurgence in the 1970s (Packer 1994). The Kurya declared their independence and pulled down a Tanzania flag, replacing it with a leopard banner. Although, the government forces ended this insurrection, the hostility between Kurya and the park including its staff is still widespread. In recent years, the conflicts in the western Serengeti Park have been intensified following the upgrading of the previously Game Controlled Areas to Game Reserves. Since December 2011, there has been an ongoing massive organized poaching of elephants inside the Tarangire National Park that has led to at least 30 elephants killed in year 2012 alone (Manendo, Park Warden-pers. comm. 2012). Although the recent surge in elephant poaching is principally driven by external market demands for ivory, involvement of local people who once tolerated the wildlife suggests waning park-local community relationships. This has come about due to increasing opportunity costs on the part of local communities, such as livestock depredation, crop damage, zoonotic diseases, damage to infrastructure and attacks by dangerous wildlife species (Rija 2009).
Globally, Tanzania is often described as a rich and stable state, though it is among the very poor countries. The country is blessed with abundant natural resources, which include forests and woodlands, wild animals, rivers, lakes and wetlands (MNRT 2012). Tanzania is also endowed with a variety and huge reserves of minerals which include Gold, Nickel, Tanzanite, Diamond, Copper, Iron ore, Coal, Limestone, Soda ash, Gypsum and Phosphate (URT 1997b). Despite the enormous resources wealth and political stability, the country’s performance economically has not been impressive.
Tanzania is classified as one of the least developed countries in the world with external aid accounting for about 40% of the national budget (Dempster 2007). The Tanzanian Poverty and Human Development Report of 2005 estimated that 36% of the population live below the ‘basic needs’ poverty line’ (URT 2005). The UN Human Development Report (2007/2008) ranked Tanzania 159th out of 177 countries. In addition, The World Bank report (2012) reveals that the percentage of the population who lived on less than $1.25 and $2 a day at 2007 international prices was estimated at 67.9 and 87.9%, respectively.
Poverty at the national level has an impact on funding of the biodiversity sector. The notable impact was observed between the 1970s and 1980s where the global economic recession and, consequently, underfunding of the sector caused rampant poaching of rhino and elephants. Poverty at household level reduces ability of people to improve on existing livelihood strategies, thus forcing them to opt for coping strategies that are unsustainable and ecologically destructive. For example, because of poverty, peasants barely can afford to purchase and use agricultural inputs to increase crop production on their lands. Food insecurity and income poverty resulting from this scenario may lead to conversion of more wildlife habitats into croplands as well as killing of wild animals for protein (Hackel 1999, Loibooki et al. 2002, Kideghesho et al. 2005, Wittemyer et al. 2008). Household poverty also limits access and usage of electricity as a source of energy. Wood fuel (firewood and charcoal) has remained the most dominant and reliable source of energy for cooking and heating, both in urban and rural areas accounting to over 90% of daily total energy consumption that is required by more than 85% of the country’s population (URT 2003). The ever increasing fuel energy demands put more woodlands areas under pressure thereby driving significant land cover change of most unprotected rangelands.
The rangelands that were mainly devoted to pastoralism and wildlife conservation had sparse human population. However, the recent population saturation in fertile and high rainfall areas together with escalating poverty have motivated in-migration to rangelands where people can access land for cultivation, though there is high risk of crop failure. Furthermore, wildlife (as a source of game meat) is a potential asset for tourism; presence of water bodies (rivers and lakes), good pastures and some mineral deposits have acted as important population pull-factors to rangelands (Baillies et al. 2004, Wittemyer et al. 2008), though such supposition is still contested (Joppa et al. 2009). A good example of this scenario is the western part of Serengeti National Park. Over the last six decades, the area had recorded a rapid demographic growth. Between 1948 and 1978, the human population in the Eastern Lake Victoria basin increased from 1.5 to 3.3 million although this growth had minimal effect on the areas adjoining Serengeti National Park (MNRT 1985). An increase of human settlements on the fertile lands found closely to Lake Victoria stimulated the movement to the periphery of the park. Between 1957 and 1967, the human population adjacent to Serengeti National park grew at the rate of 10% per annum. The natural rate of increase was 3.4% while immigration contributed the remaining 6.6% (MNRT 1985). To-date population growth around the park has continued to be a serious issue (Kideghesho et al. 2005, Wittemyer et al. 2008).
The rapid human demographic growth increases demand and competition for resources that has resulted in an increased exploitation of resources at the highest level beyond the capacity of the available resources. The demands were associated with wildlife and habitat destruction including land for settlements, cultivation and livestock grazing; plants for fuel wood, building poles, and timber; and water points for livestock and domestic use. Essentially, demographic growth is the prime cause of wildlife poaching and habitat loss (Campbell et al. 2001, Loibooki et al. 2002, Kideghesho et al. 2005).
The role of human population growth in generating conflicts in the rangelands can be summarized under the following three problems associated with living closely to the protected areas as experienced within the Serengeti ecosystem:
-
Disruption of ecological processes that are essential in maintaining biodiversity
Human impact causes depressing activities of migratory herbivores with a consequence of detrimental effects on the vegetation dynamics (McNaughton and Banyikwa 1995). Also, the disruption of migratory corridors can render the migration in the Serengeti, one of the world’s Endangered Biological Phenomena (EBP).
-
Increased hunting for home or market consumption
Poaching statistics in Serengeti and Tarangire illustrate the relationship between human population growth and its pressure on the wild resources (Campbell et al. 2001, Loibooki et al. 2002, Rija 2009).
-
Increased pressure from local people to open protected lands for community use
The expansion of cultivation and settlements forced re-alignments of the boundaries of Maswa Game Reserve for three times and thus causing 15% loss of the original area (MNRT 1985). Also, the pastoralists in some villages in Bunda and Serengeti Districts are appealing for Government to authorize the access to critical grazing and water points in Grumeti and Ikorongo Game Reserves (Kideghesho pers. obs. 2006). Manchira and Rubana Rivers in the two reserves, respectively, are critical water sources for communities who are constantly complaining on the denied access. However, these communities have been illegally admitted to access these resources due to lack of an alternative (Table 2).
Size and rate of increase of local communities and modelled number of meat hunters West of the Serengeti in Tarime, Serengeti, Musoma Rural, Bunda, Bariadi, Maswa and Meatu Districts, and Kalemela and Mkula Wards in Magu District, within 45 km from the boundary of protected area (Source: Campbell and Hofer 1995).
| Distance class (Km) | Area (Km2) | 1988 population (× 1000) | Estimated no. of hunters, 1988 (× 1000) | 1978 population (× 1000) | Estimated no. of hunters 1978 (× 1000) | Mean annual % rate of population increase | Annual % rate of increase of hunters 1978–1988 |
|---|---|---|---|---|---|---|---|
| 0–5 | 3 429 | 92.77 | 12.99 | 62.30 | 8.44 | 4.06 | 3.99 |
| 5–10 | 3 355 | 134.09 | 9.13 | 99.60 | 7.26 | 3.02 | 2.96 |
| 10–15 | 3 289 | 136.95 | 5.17 | 111.74 | 4.07 | 2.06 | 2.01 |
| 15–20 | 3 312 | 128.65 | 2.55 | 103.49 | 2.07 | 2.20 | 2.22 |
| 20–25 | 3 338 | 96.91 | 0.91 | 76.32 | 0.75 | 2.42 | 2.39 |
| 25–30 | 3 420 | 92.30 | 0.42 | 68.57 | 0.32 | 3.02 | 3.03 |
| 30–35 | 3 444 | 129.84 | 0.28 | 92.30 | 0.22 | 3.47 | 3.32 |
| 35–40 | 3 422 | 127.50 | 0.14 | 97. 84 | 0.10 | 2.68 | 2.84 |
| 40–45 | 3 449 | 116.91 | 0.80 | 83.65 | 0.06 | 3.40 | 3.47 |
| Total | 30 457 | 1055.91 | 31.66 | 795.80 | 23.29 | 2.83 | 3.11 |
| Distance class (Km) | Area (Km2) | 1988 population (× 1000) | Estimated no. of hunters, 1988 (× 1000) | 1978 population (× 1000) | Estimated no. of hunters 1978 (× 1000) | Mean annual % rate of population increase | Annual % rate of increase of hunters 1978–1988 |
|---|---|---|---|---|---|---|---|
| 0–5 | 3 429 | 92.77 | 12.99 | 62.30 | 8.44 | 4.06 | 3.99 |
| 5–10 | 3 355 | 134.09 | 9.13 | 99.60 | 7.26 | 3.02 | 2.96 |
| 10–15 | 3 289 | 136.95 | 5.17 | 111.74 | 4.07 | 2.06 | 2.01 |
| 15–20 | 3 312 | 128.65 | 2.55 | 103.49 | 2.07 | 2.20 | 2.22 |
| 20–25 | 3 338 | 96.91 | 0.91 | 76.32 | 0.75 | 2.42 | 2.39 |
| 25–30 | 3 420 | 92.30 | 0.42 | 68.57 | 0.32 | 3.02 | 3.03 |
| 30–35 | 3 444 | 129.84 | 0.28 | 92.30 | 0.22 | 3.47 | 3.32 |
| 35–40 | 3 422 | 127.50 | 0.14 | 97. 84 | 0.10 | 2.68 | 2.84 |
| 40–45 | 3 449 | 116.91 | 0.80 | 83.65 | 0.06 | 3.40 | 3.47 |
| Total | 30 457 | 1055.91 | 31.66 | 795.80 | 23.29 | 2.83 | 3.11 |
Generally, wildlife corridors play vital ecological roles in enhancing biodiversity and survival of a large number of species. In addition, the function of wildlife corridors include serving as areas of habitat, connecting wildlife populations separated by human activities (such as roads, development, or logging), facilitating the re-establishment of populations that have been reduced or eliminated due to random events (such as fires or disease), and allowing an exchange of individuals between populations, preventing the negative effects of reduced genetic diversity potentially associated with long-term population isolation (Henle et al. 2004, Frankham 1996). Also, wildlife corridors increase the area and diversity of habitats over and above the area of the two habitat patches connected.
Wildlife corridors, however, are under serious threat. First, there is human population pressure attributed to a number of population-pull factors in the rangelands and push-factors in the areas of high agricultural potential. Secondly, there is lack of by-laws to protect the corridors against unsustainable use and activities that are incompatible with biodiversity conservation. Lake Manyara Basin is one of the areas, which have been experiencing an increasing population pressure. The major population pull-factors at this area include demand for agricultural land, construction of Minjingu Phosphate factory, establishment of fishing camps, small mining activities (at Marang Forest Reserve), growth of tourism, and other economic opportunities. Population push factors from the areas with acute land shortage, such as Kilimanjaro region, have also affected the lake Manyara basin. The major outcome of all the identified factors is an increased threat in the existing five wildlife corridors, which provide ecological links between Lake Manyara National Park and outside systems (Jones et al. 2009) as revealed in Table 3.
Threats facing five wildlife corridors linking Lake Manyara NP and outside systems (Sources: Shemweta and Kideghesho 2000; Jones et al. 2009).
| Corridor | Link protected area | Key species | Human threats |
|---|---|---|---|
| Kwakuchinja-Mbugwe Wildlife Corridor | Tarangire National Park | Zebra and Wildebeest | Settlements and crop cultivation |
| Mayoka-Magara-Mwada-Vilima Vitatu | Tarangire National Park | Buffalo and Eland | Cotton field expansion in Mwada |
| Jangwani | Mto wa Mbu Game Controlled Area | Zebra and Wildebeest | Settlements, cultivation and campsites |
| Upper Kitete-Lositete | Ngorongoro Conservation Area | Elephant, Buffalo, Hippos | Intensive crop cultivation mainly maize and wheat. |
| Laja | NCA and Marang Forest | Elephants | Livestock grazing, deforestation, mining |
| Corridor | Link protected area | Key species | Human threats |
|---|---|---|---|
| Kwakuchinja-Mbugwe Wildlife Corridor | Tarangire National Park | Zebra and Wildebeest | Settlements and crop cultivation |
| Mayoka-Magara-Mwada-Vilima Vitatu | Tarangire National Park | Buffalo and Eland | Cotton field expansion in Mwada |
| Jangwani | Mto wa Mbu Game Controlled Area | Zebra and Wildebeest | Settlements, cultivation and campsites |
| Upper Kitete-Lositete | Ngorongoro Conservation Area | Elephant, Buffalo, Hippos | Intensive crop cultivation mainly maize and wheat. |
| Laja | NCA and Marang Forest | Elephants | Livestock grazing, deforestation, mining |
The blockage of wildlife corridors linking Lake Manyara National Park and other areas has led to some undesirable ecological impacts. The biggest impact is the reduced population and local extinction of some large mammal species, both within the park and along the corridors (Newmark 1996). However, the impact of other factors including poaching should not be underestimated. A study by Gamassa (1989) on the Wildlife Corridor at Kwa Kuchinja Mbugwe (KWC) indicated that there is a 72% decline of species diversity of large mammals along KWC. Boshe (1989) in Hassan (1998) uncovered that seven species that were previously regarded as regular users of the KWC were locally extinct: cape eland (Tragelaphus oryx), hartebeest, (Alcelaphus buselaphus), buffalo (Syncerus caffer), oryx (Oryx gazella), lesser kudu (Tragelaphus imberbis), cheetah (Acynonyx jubatus), and leopard (Panthera pardus). In the Lake Manyara National Park, the following nine species were reported to be locally extinct: African wild dog (Lycaon pictus), cape eland (Tragelaphus oryx), hartebeest, oribi (Ourebia ourebi), black rhinoceros (Diceros bicornis), lesser kudu, cheetah, mountain reedbuck (Redunca fulvorufula) and common reedbuck (Redunca arundinum) (Hassan 1998, Kideghesho 2001).
The HIV/AIDS problem has emerged as one of the worst pandemics in history. The pandemic has some undesirable impacts to virtually all the sectors and parts of Tanzania. The problem has caused an increase of orphan children, the breaking of families and marriages, a rise in poverty and the increased disappearance of labour force. The wildlife sector is by no way exempted from this scenario. Although there are no empirical data that quantify the impact of the pandemic on the wildlife sector, some reports (e.g. Ngoti and Baldus 2004) show existing or potential influences.
The fact that HIV/AIDS exacerbates poverty implies that people are compelled to adopt certain strategies that will enable them to cope with the impacts of poverty. The most accessible strategies in the rangelands entail illegal and/or unsustainable use of natural resources (viz. wild foods, wildlife, medicinal plants, timber and fuel wood). Furthermore, the pandemic lowers the efficiency of managing and enforcing conservation laws. The impacts of HIV/AIDS pandemic on biodiversity can be explained by the following mechanisms.
An increased poaching of wildlife to meet subsistence and income needs: HIV/AIDS pandemic has caused many deaths and debilitation to families and economies in Tanzania. Ultimately, scourge has made natural resources become the main source of income generation to substitute other lost income earning opportunities (Ngoti and Baldus 2004, Thaxton 2007). As breadwinners die, orphans opt for poaching as a more viable strategy for survival through meeting subsistence needs and income to cater for other needs including medical services (Thaxton 2007).
Increased poaching to cater for health needs: For example, the poaching of giraffe has never been an issue that has drawn significant conservation or management attention in the past; but recently, the poaching of giraffe has been widely observed in Tanzania. A critical good example is the mass poaching of giraffes at Monduli District and the West Kilimanjaro Wildlife corridor (striding between Arusha and Kilimanjaro National Parks) in the period between 2004 and 2008, which was fuelled by the beliefs of traditional healers (witch doctors) that brain and bone-marrow of a giraffe could cure HIV-AIDS (Anon. 2004, Anon. 2010).
Increased and unsustainable rates of harvesting medicinal plants to treat some HIV-associated opportunistic diseases: HIV/AIDs pandemic and associated opportunistic diseases, such as tuberculosis, high blood pressure, and diabetes, have increased overexploitation of some species because such species are believed to bear a medicinal value. For example, the recent human population influx at Samunge Village, Loliondo (Kwa Babu) where thousands of people from all over East Africa have been attracted for herbal concoction from a shrub Carissa edulis, which is believed to treat Herpes simplex according to Tolo et al. (2010). In addition to overexploitation of these species, environmental impacts, such as pollution due to littering of human wastes and plastics and habitat degradation due to increased deforestation for firewood as well as physical impacts of vehicles were apparent (Figure 2).
Top left: People on their way to Loliondo-Samunge village for the dosage of the said miracle cure; Bottom left: Thousands of people to and from the Loliondo-Samunge village; Top right: Retired Pastor Ambilikile Mwasapile giving dosage of the medicine to patients; Bottom right: People at Loliondo-Samunge village waiting for the dosage of the miracle medicine.
Top left: People on their way to Loliondo-Samunge village for the dosage of the said miracle cure; Bottom left: Thousands of people to and from the Loliondo-Samunge village; Top right: Retired Pastor Ambilikile Mwasapile giving dosage of the medicine to patients; Bottom right: People at Loliondo-Samunge village waiting for the dosage of the miracle medicine.
Increased rates of illnesses and deaths among park rangers, senior officials, community game guards and other conservation personnel have ultimately weakened the performance of the sector. This is likely to be the case because wildlife staffs are likely to fail to execute their duties including law enforcement in case they fall sick. Also, poachers may take advantage of this situation and poach when wildlife staff members are looking after their sick relatives or attending funerals. Economically, HIV/AIDS pandemic imposes huge financial costs to government, conservation agencies and communities.
Besides harbouring biodiversity resources and supporting livestock production, the macro- and micro-economic potentiality of rangelands is still untapped in Tanzania. Among the potential resources in the rangelands are mineral deposits. The reality that mining activities in the rangelands cause severe environmental destruction cannot be questioned and this has prompted concern of the conservationists and the general public. The most recent debate on this issue revolves around the government plans to grant licence for uranium mining at the area between the Selous Game Reserve and Selous-Niassa Wildlife Corridor. The area is exceptionally rich in wildlife species including elephant (Loxodonta africana), buffalo, eland, sable antelope (Hippotragus niger), hippo (Hippopotamus amphibius), Lichtenstein hartebeest (Alcelaphus lichtensteinii), common waterbuck (Kobus ellipsiprymnus), bushbuck (Tragelaphus scriptus), common duiker (Sylvicapra grimmia), common reedbuck, wildebeest (Connochaetes taurinus), zebra (Equus burchellii), impala (Aepyceros melampus), klipspringer (Oreotragus oreotragus), warthog (Phacochoerus aethiopicus), bush pig (Potamochoerus larvatus), leopard and lion (Pathera leo), spotted hyena (Crocuta crocuta), jackal (Canis aureus) and civet (Civettictis civetta). Several packs of wild dogs are observed in all parts of the corridor.
Despite its biodiversity and wildlife potential, the Selous–Niassa Wildlife Corridor is threatened by an increasing human population and activities, which are incompatible with conservation interests. The most recent menace is likely to come from the mining activities following the prospects of the three international mining companies namely, Mantra, Uranex and Uranium Resources. The environmental implications that are likely to arise from this economic opportunity include: blockage of the wildlife corridor and interference with migratory routes of animals and acting as a population pull factor to the area. The latter may have as a consequence a) an increase of pressure on the natural resources and potentially more illegal logging, cultivation and poaching, b) loss/disturbance of biodiversity due to vegetation clearance, disturbance to biodiversity through blanketing of vegetation cover, c) increased potential for accidents to wildlife and people, d) health impacts to fauna from the drinking of contaminated water and from heavy metals taken up with forage, and e) potential for accidents to animals falling in un-rehabilitated pits.
Further, Tanzania’s government has also implemented or allowed implementation of a number of development projects in the rangelands, which had proved (or are likely) to be detrimental to biodiversity. Examples include the following;
-
Construction of Tanzania-Zambia Railway (TAZARA) in 1970s.
The project had caused the fragmentation of Selous Game Reserve (SGR) and Magombera Forest Reserve (MFR) (Maganga 1994). Unlike MFR, the impact of the railway on SGR was less visible because of its large area. The MFR had 15 km2; however, about 50% of its area was reduced by ILLOVO sugar cane Company, thus lowering its conservation effectiveness (Marshall 2005). Given its ecological importance as the critical habitat for an endangered sub-species of red colobus monkey (Colobus badius gordonorum), reptiles and amphibians (Menegon et al. 2009), the MFR had to be annexed to SGR as a measure for improving its conservation effectiveness (Baldus 1992).
-
Investment policies, which allowed the construction of tourist hotels and lodges in the northern tourist circuit in the 1990s.
These were deliberate efforts by the government towards improving the country’s economy through the game viewing tourism. However, these policies had some negative impacts on wildlife. Some of the hotels were built on the wildlife migratory routes and water catchment areas, for example: Sopa Hotel in Ngorongoro Conservation Area and Serena Hotel on the rim of Lake Manyara National Park (Runyoro, pers. comm.).
-
The Proposed Mto wa Mbu-Mugumu road passing through Serengeti National Park.
Other than acting as a big population pull factor to Serengeti area (see impacts of population growth in sections 2.4 and 2.5 above), it may directly affect biodiversity through clearing of vegetation, road kills and blockage of the migratory corridor for wildebeest, Grant’s gazelle and zebra moving between Serengeti and Maasai-Mara National Reserve in Kenya. The lessons from Mikumi National Park and other protected areas elsewhere where public roads pass across indicate negative effects and ecological impacts associated with roads. Drews (1995) reports that over 50 different animal species including endangered species have been killed by road accidents at Mikumi national park just within a two-year period of the field study. Furthermore, the author estimated a minimum of 3 kills per day during the same period. Similarly, various animal species were concentrating in some areas; avoiding habitats close to the road. This suggests some negative ecological impacts roads have on the animals in Mikumi National Park (Newmark 1996). These data suggest that the inception of the proposed road through the Serengeti area will have consequences such as increasing animal physiological stresses, mortality and sustainability of the ecosystem (Lunde 2013, Fyumagwa et al. 2013).
-
Proposed Lake Natron Soda Ash Plant
A proposal by Tata Chemical Industries Ltd in collaboration with the Tanzanian Government to construct a $450 million factory that would produce 500, 000 tonnes of soda ash per year and employ 150 permanent staff sounds economically promising. However, its ecological impacts cannot be underestimated.
Lake Natron is the only regular breeding site for Lesser Flamingos (Phoenicopterus minor) in the Eastern part of Africa. The 1.5–2.5 million Lesser Flamingos represents three quarters of the world population. The area is isolated and undisturbed and has adequate food and nesting sites for flamingos. It is both an Important Bird Area and a Ramsar Site. Also, the project may cause a negative impact on mammal populations and vegetation in the northern area of Gelai to Longido. In addition, the opening of the area to hundreds of workers may give rise to the bush meat and charcoal trade.
Climate change is increasingly being recognized as a global crisis threatening human survival and biological resources. There is growing evidence that climate change, particularly increasing temperatures, is already having significant impacts on the world’s physical, biological and human systems, and it is expected that these impacts will become more severe in the future (Gitay et al. 2002, Balmford et al. 2003, de Wit and Stankiewicz 2006, Wilson and Maclean 2011). Studies suggest that many plants and animals are unlikely to survive within uncertain climate change limits (Thomas et al. 2004, Maclean and Wilson 2011). By 2050, climate change will lead to the extinction of 15–37% of a total sample of 1, 103 land plants and animals (Thomas et al. 2004). In Tanzania, the impacts of climate change have been felt in virtually all ecosystems, including the rangelands. For instance, the severe droughts in the 1990s and 2000s had forced the pastoralists to shift their herds towards southern Tanzania in search of pastures. This had led to the destruction of habitats, reduced biodiversity, and destruction of water sources as observed in Ihefu and Great Ruaha River (Kashaigili et al. 2009).
In their book – Serengeti 111: Human Impacts on Ecosystem Dynamics – Sinclair et al. (2008) predicted the impacts the anthropogenic activities and natural changes will exert on the global climate and atmospheric chemical composition over the next five decades. They contended that even in the absence of local anthropogenic activities, the risk to the isolated and complex ecosystems like Serengeti will be extremely high. An alteration of vegetation, hydrology, quality of forage to herbivores, species diversity, migration patterns, disease outbreaks to human, fauna and flora, change or destruction of habitats, among others, are potential impacts envisaged from high carbon emissions into the atmosphere. These changes have direct consequences for the health of the Serengeti ecosystem (Sinclair et al. 2008).
The rise of temperature and change of rainfall patterns in Serengeti provide further illustration of the impacts of climate change on the biodiversity. Studies have shown that the temperature at Amboseli and areas neighbouring Serengeti have increased by 0.275°C per annum between 1976 and 2000 (Altmann et al. 2002). In the recent years the flow of the Mara River, which cuts right across Serengeti National Park, has become increasingly inconsistent (Mango et al. 2011) raising concerns over the health functioning of the Serengeti ecosystem. Using a simulation model, Sinclair et al. (2008) predicted the potential effects that average annual precipitation and changes in the precipitation variables will bear on the wildlife, humans and livestock numbers. These predictions are summarized in Table 4. The impacts of climate change on biodiversity may be manifested indirectly through exacerbating other factors or agents contributing to the loss of biodiversity. The factors include poverty, which may force the victims to adopt coping strategies which are destructive to biodiversity, such as illegal hunting and encroachment (e.g. Loibooki et al. 2002), wildfire, human-wildlife conflicts, and soil erosion and siltation of water bodies that may increase eutrophication of lakes thereby impacting aquatic and terrestrial wildlife negatively.
Predicted effects of mean precipitation (a) and changes in variability of precipitation (b) in relation to wildebeest population, hunting offtakes and human and livestock population. Figures reported are steady-state values at the end of a 50-year simulation
| a) Predicted effects of changes in mean annual rainfall | |||
| Base case | Increase in mean rainfall | Decrease in mean rainfall | |
| Mean annual rainfall (mm/yr) | 830 | 1 200 | 400 |
| Wildebeest population: | |||
| Resident population | 14 890 | 21 450 | 28 330 |
| Migrating population | 1 257 000 | 1 809 000 | 613 500 |
| Hunting offtake: | |||
| Resident population | 55 | 81 | 5 489 |
| Migrating population | 20 690 | 30 890 | 9, 971 |
| Human population | 135 700 | 253 800 | 68 020 |
| Livestock number | 80 050 | 113 600 | 0 |
| b) Predicted effects of changes in the variance of rainfall | |||
| Base case: no variance | Moderate rainfall variance | Moderate variance with persistence | |
| Standard deviation of annual rainfall | 0 | 176 | 176 |
| Persistence of deviation | 0 | 0 | 0.5 |
| Wildebeest population: | |||
| Resident population | 14 890 | 32 870 | 21 260 |
| Migrating population | 1 257 000 | 1 173 300 | 1 196 000 |
| Hunting offtake: | |||
| Resident population | 55 | 5 125 | 1 896 |
| Migrating population | 20 690 | 19 890 | 19 950 |
| Human population | 135 700 | 159 150 | 147 830 |
| Livestock number | 80 050 | 7 188 | 32 950 |
| a) Predicted effects of changes in mean annual rainfall | |||
| Base case | Increase in mean rainfall | Decrease in mean rainfall | |
| Mean annual rainfall (mm/yr) | 830 | 1 200 | 400 |
| Wildebeest population: | |||
| Resident population | 14 890 | 21 450 | 28 330 |
| Migrating population | 1 257 000 | 1 809 000 | 613 500 |
| Hunting offtake: | |||
| Resident population | 55 | 81 | 5 489 |
| Migrating population | 20 690 | 30 890 | 9, 971 |
| Human population | 135 700 | 253 800 | 68 020 |
| Livestock number | 80 050 | 113 600 | 0 |
| b) Predicted effects of changes in the variance of rainfall | |||
| Base case: no variance | Moderate rainfall variance | Moderate variance with persistence | |
| Standard deviation of annual rainfall | 0 | 176 | 176 |
| Persistence of deviation | 0 | 0 | 0.5 |
| Wildebeest population: | |||
| Resident population | 14 890 | 32 870 | 21 260 |
| Migrating population | 1 257 000 | 1 173 300 | 1 196 000 |
| Hunting offtake: | |||
| Resident population | 55 | 5 125 | 1 896 |
| Migrating population | 20 690 | 19 890 | 19 950 |
| Human population | 135 700 | 159 150 | 147 830 |
| Livestock number | 80 050 | 7 188 | 32 950 |
Source: Sinclair et al. (2008)
Next to habitat destruction and fragmentation, invasive alien species are among the world’s most significant threats to indigenous biodiversity, their introduction and establishment will ultimately lead to severe leveling off of biodiversity. These species are increasingly spreading both in natural and non-natural systems (McNeely et al. 2001). Many rangelands of Tanzania including national parks and other forms of protected areas have also not been immune to infestation by invasive species (Foxcroft et al. 2006). As a consequence, the invasive species have now been recognized in the conservation agendas countrywide. The most important areas that are highly infested by these species include the Ngorongoro Conservation Area Authority, Serengeti National Park, and a number of other non-protected areas. The available literature shows that invasive alien species continue toengulf grazing lawns of the Ngorongoro crater (Henderson 2002). These include Datura stramonium, Acacia mearsii, Caesalpinia decapetala, Eucalyptus camaldulensis, Lonicera japonica, Argemone mexicana. At the Serengeti National park the invasive species Cylindropuntia exaltata, Opuntia stricta var. dillennii, Opuntia monocantha and Pistia stratiotes remain a significant threat to the ecosystem (Foxcroft 2003). The major impacts of the invasive species include disruption of the general ecology of an ecosystem, changing the fire regime, water and nutrient cycling and affecting the bio-geochemical processes of landscapes (Cronk and Fuller 1995).
Theories of invasion predict increasing invasiveness with increasing habitat disturbances (Vermeij 1996, Williamson 1999, Davis et al. 2000) as well as global climatic change (Dukes and Mooney 1999, Kolar and Lodge 2001). There have been increasing habitat disturbances in most protected areas cores and edges due to livestock grazing. For example, a recently annexed Ihefu to Ruaha National Park is potentially a victim of invasive species that in future may invade other parts of the park. In Mkomazi National Park in northern Tanzania, past livestock grazing at the area may have facilitated occurrence of undesirable plant species into the park (Homewood and Brockington 1999). Parthenium hysterophorus is one of the most serious invasive alien species that is already a threat to Ethiopian rangelands and is spreading southward into the East African countries (McNeely et al. 2001). In Tanzania, this species has been observed mostly in the urban landscapes (Rija pers. obs. 2011) and along roadsides of the countryside (Klark pers. comm. 2011). Although the population size of the species in most areas is still low, the species has the ability to dramatically increase and spread widely un-noticed, potentially affecting biological diversity in rangeland ecosystems. Further, edge encroachment is still a big challenge for many national parks because of an illegal grazing that may introduce invasive species from other areas outside. The mounting pressures on the rangelands due to the growing human population coupled with climate change impact are set to affect rangeland ecosystems even more. In this respect, the future of the Tanzanian rangelands remains uncertain.
Civil wars are a salient feature in Africa. Unlike many other African countries, Tanzania had never experienced such wars; however, the country has felt the impact of these wars. The country has been surrounded by conflicts and hosted refugees from Democratic Republic of Congo, Burundi, and Rwanda. The number and lifestyle of refugees have caused some notable environmental and ecological problems particularly in the areas occupied by refugee camps. The number of refugees in Tanzania was about 1.2 million in 1994; this is the largest number in Africa compared to all other countries (The Citizen, Wednesday September 29 2010). Refugees brought with them sophisticated equipment including automatic weapons that were readily available for conducting criminal acts including illegal hunting of wildlife. This big population has created an increased demand for the rangeland resources including firewood, medicinal plants and wild meat. The two most-hard hit regions by the refugee saga include Kigoma and Kagera regions particularly in Moyowosi-Kigozi and Burigi-Biharamulo game reserves respectively, where refugees were and are still housed in camps. Currently however, there is a state repatriation order for all illegal immigrants to their home countries. The outcome of the influx of refugees was habitat destruction and illegal hunting of wildlife, which led to a drastic decline in population of 13 wild ungulates by almost 90% in Burigi-Biharamulo Game Reserves (Table 5). In this reserve, animals like topi (Damaliscus korrigum), giraffe, buffalo, eland and other medium to small sized animals including roan and sable antelopes, impala, warthogs and zebra have been severely depleted within just a decade, between 1990 and 2000 (Stoner et al. 2007). Some species, such as sitatunga (Tragelaphus spekei) and sable antelope are feared to have gone extinct in the same reserve. Similarly, bushbuck, sitatunga, warthog, buffalo and impala showed persistent population declines at Moyowosi-Kigozi game reserve, an important rangeland in western Tanzania during the same decade. However, the population of some animals such as zebra elephant, giraffe (Giraffa camelopardalis), reedbuck and topi have shown a slight increase and they were relatively stable in this reserve (Stoner et al. 2007).
Trends in major species of animal populations in the Burigi Game Reserve 1990–2000 (Source: TWCM 1990, 1998, Jambiya et al. 2007). D* = Population declined and NC* = No change, according to Stoner et al. (2007).
| Animal species | Burigi Game Reserve | Moyowosi-Kigosi Game Reserve | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1990 Estimates | 1998 Estimates | 2000 Estimates | Trend status | 1990 wet season | 1994 wet season | 1998 wet season | Trend status | |||
| Buffalo Synceros caffer | 2670 ± ? | 44 ± ? | 78 ± 41 | D* | 7070 ± 4790 | 6652 ± 3666 | 6926 ± 3778 | D* | ||
| Bushbuck Tragelaphus scriptus | 229 ± 33 | 18 ± 15 | 153 ± 194? | D* | - | 197 ± 72 | 65 ± 36 | NC* | ||
| Eland Tragelaphus oryx | 878 ± 336 | 237 ± 102 | - | D* | - | - | - | - | ||
| Elephant Loxodonta africana | - | - | - | - | 392 ± 376 | 1583± 700 | 2262 ± 716 | I* | ||
| Giraffe Giraffa camelopardalis | 127 ± 79 | 300 ± 119 | 75 ± 27 | NC | 1043 ± 292 | 1465 ± 246 | 1131 ± 302 | NC* | ||
| Hartebeest Alcelaphus lichtensteini | 324 ± 137 | 0 | - | D* | 549 ± 190 | 1112 ± 237 | 512 ± 133 | NC* | ||
| Hippo Hippopotamus amphibius | - | - | - | - | 1518 ± 680 | 784 ± 271 | 574 ± 196 | NC* | ||
| Impala Aepyceros melampus | 5, 130 ± ? | 2, 795 ± 801 | 1157 ± 289 | D* | - | - | - | - | ||
| Reedbuck Redunca redunca | 147 ± 49 | 98 ± 31 | 84 ± 16 | D* | 486 ± 59 | 5168 ± 674 | 1524 ± 152 | NC* | ||
| Roan Antelope Hippotragus equinus | 466 ± 169 | 15 ± 15 | - | D* | - | 1738 ± 381 | 617 ± 359 | NC* | ||
| Sable Antelope Hippotragus niger | 279 ± 125 | 32 ± 20 | 9 ± 7 | D* | - | 985 ± 272 | 242 ± 146 | NC* | ||
| Sitatunga Tragelaphus spekei | 490 ± 128 | 0 | 0 | D* | 310 ± 99 | 512 ± 85 | 32 ± 20 | D* | ||
| Topi Damaliscus korrigum | 6, 399 ± 298 | 160 ± 109 | 74 ± 37 | D* | 1803 ± 773 | 9410 ± 3488 | 5061 ± 772 | NC* | ||
| Waterbuck Kobus ellipsiprymnus | 822 ± 218 | 94 ± 61 | - | D* | 835 ± 228 | 920 ± 153 | 437 ± 141 | NC* | ||
| Warthog Phacochaerus aethiopicus | 2, 628 ± 188 | 71 ± 61 | 54 ± 40 | D* | 1137 ± 237 | 1251 ±143 | 299 ±118 | NC* | ||
| Zebra Equus burchelli | 6, 552 ± 1, 127 | 606 ± 140 | 656 ± 147 | D* | 1412 ± 618 | 3971 ± 1830 | 787 ± 248 | NC* | ||
| Animal species | Burigi Game Reserve | Moyowosi-Kigosi Game Reserve | ||||||
|---|---|---|---|---|---|---|---|---|
| 1990 Estimates | 1998 Estimates | 2000 Estimates | Trend status | 1990 wet season | 1994 wet season | 1998 wet season | Trend status | |
| Buffalo Synceros caffer | 2670 ± ? | 44 ± ? | 78 ± 41 | D* | 7070 ± 4790 | 6652 ± 3666 | 6926 ± 3778 | D* |
| Bushbuck Tragelaphus scriptus | 229 ± 33 | 18 ± 15 | 153 ± 194? | D* | - | 197 ± 72 | 65 ± 36 | NC* |
| Eland Tragelaphus oryx | 878 ± 336 | 237 ± 102 | - | D* | - | - | - | - |
| Elephant Loxodonta africana | - | - | - | - | 392 ± 376 | 1583± 700 | 2262 ± 716 | I* |
| Giraffe Giraffa camelopardalis | 127 ± 79 | 300 ± 119 | 75 ± 27 | NC | 1043 ± 292 | 1465 ± 246 | 1131 ± 302 | NC* |
| Hartebeest Alcelaphus lichtensteini | 324 ± 137 | 0 | - | D* | 549 ± 190 | 1112 ± 237 | 512 ± 133 | NC* |
| Hippo Hippopotamus amphibius | - | - | - | - | 1518 ± 680 | 784 ± 271 | 574 ± 196 | NC* |
| Impala Aepyceros melampus | 5, 130 ± ? | 2, 795 ± 801 | 1157 ± 289 | D* | - | - | - | - |
| Reedbuck Redunca redunca | 147 ± 49 | 98 ± 31 | 84 ± 16 | D* | 486 ± 59 | 5168 ± 674 | 1524 ± 152 | NC* |
| Roan Antelope Hippotragus equinus | 466 ± 169 | 15 ± 15 | - | D* | - | 1738 ± 381 | 617 ± 359 | NC* |
| Sable Antelope Hippotragus niger | 279 ± 125 | 32 ± 20 | 9 ± 7 | D* | - | 985 ± 272 | 242 ± 146 | NC* |
| Sitatunga Tragelaphus spekei | 490 ± 128 | 0 | 0 | D* | 310 ± 99 | 512 ± 85 | 32 ± 20 | D* |
| Topi Damaliscus korrigum | 6, 399 ± 298 | 160 ± 109 | 74 ± 37 | D* | 1803 ± 773 | 9410 ± 3488 | 5061 ± 772 | NC* |
| Waterbuck Kobus ellipsiprymnus | 822 ± 218 | 94 ± 61 | - | D* | 835 ± 228 | 920 ± 153 | 437 ± 141 | NC* |
| Warthog Phacochaerus aethiopicus | 2, 628 ± 188 | 71 ± 61 | 54 ± 40 | D* | 1137 ± 237 | 1251 ±143 | 299 ±118 | NC* |
| Zebra Equus burchelli | 6, 552 ± 1, 127 | 606 ± 140 | 656 ± 147 | D* | 1412 ± 618 | 3971 ± 1830 | 787 ± 248 | NC* |
Illegal hunting of wildlife remains a persistent threat to the wildlife across the country. Despite poaching becoming increasingly high and widespread, its impact on the wildlife populations has not caught the attention of policy makers as it is assumed to be minimal (Barnett 2000). This is partly because many rangelands experiencing intensive poaching remain un-researched because the majority of them fall outside protected areas. Also, the available literature are sporadic and biased towards certain geographic locations and protected ecosystems particularly Serengeti (e.g. Hofer et al. 2000, Loibooki et al. 2002, Marealle et al. 2010) and Katavi (Caro 2008, Martin and Caro 2012) leaving other equally impacted ecosystems, such as Tarangire, Mikumi, and Ruaha, under-researched. Illegal hunting is a big problem in the Simanjiro plains, a seasonal refuge for wildlife dispersing from Manyara and Tarangire National Parks (Rija 2009). In our recent field visits (June, 2013) in some villages, Misima, Msomela, Mbagwe and Kinkwembe in Handeni District in north-eastern Tanzania, we were surprised with the huge number of illegally killed animals brought in the villages. At Misima village alone, 15-20 animals per day were landed in a local black market (Rija and Mwamende pers. obs. 2013) with similar such cases occurring around Swagaswaga (Madulu 2001) and Kiteto rangelands, respectively in central and northern Tanzania. These data suggest that the extent of illegal hunting is higher than previously known. Moreover, unregulated legal hunting poses an additional threat to the wildlife population. Many rangelands that support legal hunting have experienced significant declines due to uninformed excessive quota allocated to them and from unscrupulous hunters who kill in excess of their allocated quotas (Baldus and Cauldwell 2004). Controlling resident legal hunting is especially difficult because many local wildlife offices are particularly understaffed, thus most hunting goes unsupervised resulting in more animals killed than is indicated on the hunting permits (Rija 2009). Coupled with the selective nature of sport hunting (Caro et al. 2009), both illegal hunting and local licensed hunting have the potential to drive individual species to population decline (Stoner et al. 2007, Caro 2008) and local extirpation (Rija 2011) with unknown consequences on the ecosystem functions of the rangelands.
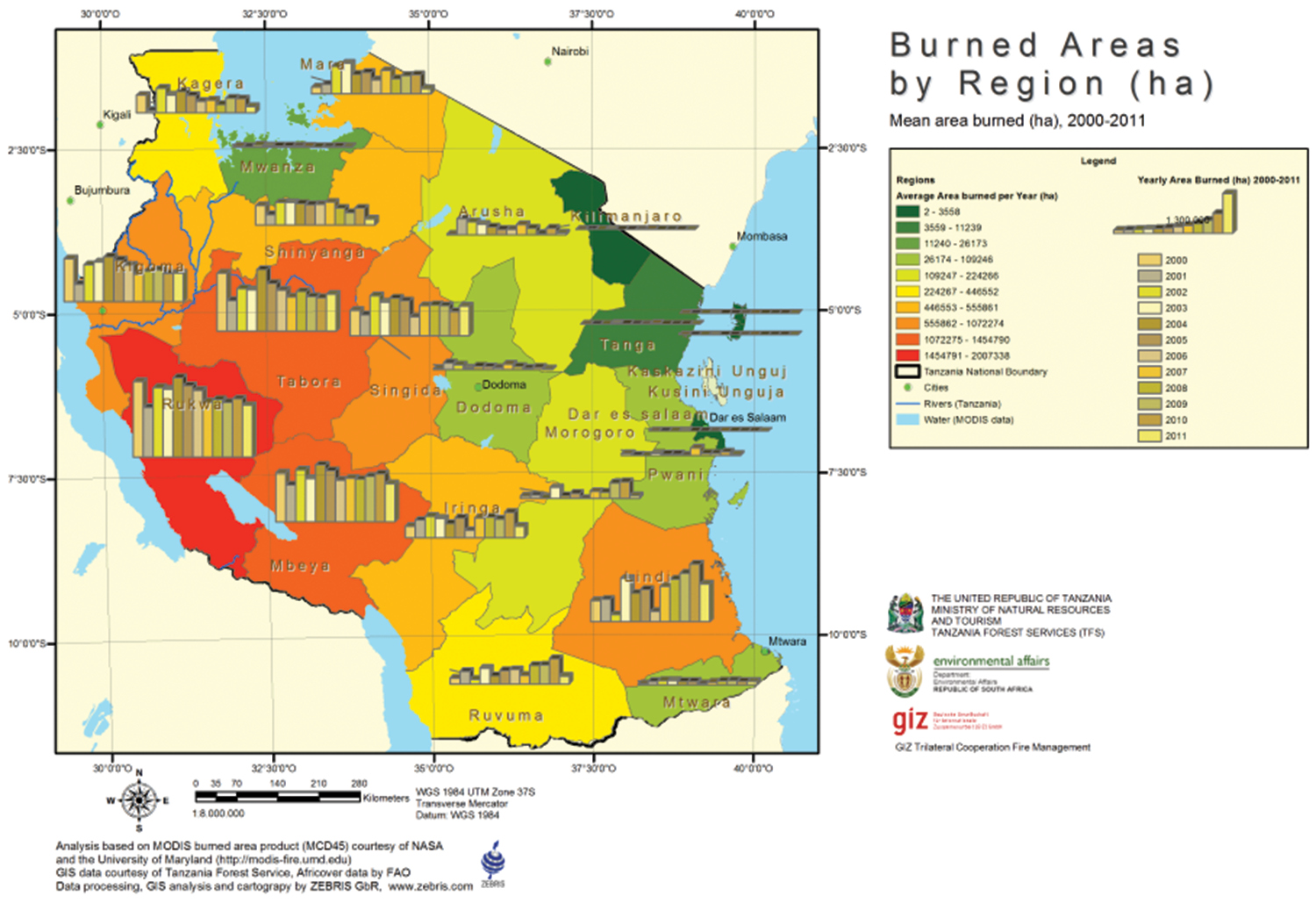
Wildfires are perhaps the most driving force of rangelands dynamics but one that remains under-appreciated by the government policies probably because of lack of empirical information. There is no fire policy in existence and fire issues are dealt on ad-hoc basis by individual ministerial sectors particularly in the ministries responsible for managing natural resources (wildlife, forests and livestock). Preliminary analysis of eleven years (from 2000–2011) of burned areas indicates however that the mainland Tanzania loses over 11 million ha of forests and woodlands annually (Rucker and Tiemann 2012). About 70% of burned area in Tanzania is woodlands and shrubland cover types, most of which fall under protected areas (national parks, game reserve and game controlled area) constituting more than 8 million ha burned annually (Rucker and Tiemann 2012). Although some of these burns are captured during prescribed burning to manage habitats by park and reserve managers, increasing evidence point out that most late blazes are caused by local communities (Butz 2009). The most fire affected rangelands are located in Katavi ecosystem, Lindi, Rukwa, Tabora, Mbeya and most western regions (Fig. 3). A task force investigating factors contributing to the significant burn statistics in these regions found that traditional hunting of rats done by resident communities (in Rukwa and Katavi regions), illegal hunting of wildlife, farming practices and arsonism contribute significantly to most wildfire incidences (NTF 2012). Fire havoc causes countless loses of biotas, human lives, ecological services and changes of local climates. The actual effects of wildfire on Tanzania’s biodiversity are difficult to understand, however, given that most such incidences go unmonitored. The government of Tanzania has welcome the report by Rucker and Tiemann (2012) and a task force on integrated fire management (under the Ministry of Natural Resources and Tourism) is working towards formulating a unified fire policy for Tanzania. This is a positive step towards controlling wildfire and its impacts on the rangeland biodiversity in the country.
Map of Tanzania showing distribution of wildfires across the country. Wildfires destroy thousands of hectars of miombo woodlands and forests killing an unknown numbers of species and threatening the functioning of ecosystems (Map adapted from Rucker and Tiemann 2012).
Unsustainable land use and associated land cover changes continue to influence on the dynamics of Tanzania rangelands’ resources. However the paucity of information on the extent and intensity of deforestation across the miombo and savannah biomes make it particularly more difficult to curtail the problem. Limited access to electrification for the majority of the human population in rural areas (about 80%) means that high energy demands are met through clearing forests and woodlands for biomass fuel (URT 2003). About one million tonnes of charcoal is burnt in Tanzania annually, with almost 70% consumed in the city of Dar es Salaam alone (Msuya et al. 2011). Demands for biomass energy claims in excess of 150 ha of forests and woodlands each year (Msuya et al. 2011) driving forest land cover change along the highway and near the city (Ahrends et al. 2010). However, these data on woodland deforestation are only indicative and may not reflect actual situation on a countrywide scale given that charcoal and firewood is consumed in significant amounts across all other Tanzanian cities. Further, in some parts of Tabora and southern (Iringa and Mbeya) regions deforestation stands at >3% annually in the miombo woodlands (Mangora 2005) because of shifting cultivation and excessive use of woods for curing tobacco (Sauer and Abdallah 2007). For example, the land converted from natural vegetation to cultivated land increased to 11.2% (between 1995 and 2000) from 4.7% (between 1984 and 1995) in some parts of Tabora (Yanda 2010). Such land use change has severe consequence on local biodiversity (Mangora 2005, Sauer and Abdallah 2007) as well as on local climates. Furthermore, clearing of woodlands in protected area is to a great extent instigated by brick burning, lumbering, charcoal making (e.g. in Swagaswaga Game Reserve, Madulu 2001) and agricultural expansion near national park boarders (Mwamfupe 1998, Vanderpost 2006, Wittemyer et al. 2008). Deforestation and habitat loss, if not checked, may have far reaching impacts on species survival and ecological functioning of protected areas (Newmark 1996, 2008).
The rangelands play critical roles in human survival and development. They support a variety of species of economic as well as socio-cultural and ecological importance. However, there are numerous challenges facing biodiversity conservation in rangelands. This paper has uncovered these challenges and attempts to develop effective measures of addressing them. Hereunder, we recommend some measures to address these challenges.
Human-wildlife conflicts should be an important issue on the policy agenda in the management of rangeland biodiversity. Most of the conflicts are a consequence of the prohibitive and restrictive policies. Transforming biodiversity resources such as wildlife from a liability to an asset, the communities will be motivated to align their behaviour with conservation goals. Further, local communities should be actively involved in the decision-making and planning of conservation including the development-related interventions. This will greatly reduce the conflicts and poverty.
Conservation education with urban and rural communities should be emphasized. Failure of implementation of conservation strategies has been partly because of the limited awareness of the people of the role of biodiversity in ecosystem and human health and limited financial resources. Conservation education may help re-align the people’s minds toward protection of biodiversity and thus conservation would trickle down from people’s own initiatives. Transforming communities into conservators requires clear understanding of the value that nature and the consequences of having non-functional ecosystems.
Poverty is one of the root causes of the biodiversity loss, and thus should be tackled. Those who destroy biodiversity in order to survive should be provided with adequate alternative livelihood strategies. The current conservation policies seeking to empower local communities economically are encouraging, but their implementation is yet to engender the expectations. The scientific studies that will lead to understanding of and, therefore, addressing the impediments towards thwarting poverty reduction effort is key to rectifying the deficiencies towards prosperity. Critical to sound poverty reduction strategies is to maximize good governance through (i) directly supporting the participatory pro-poor policies, (ii) facilitation of sound macroeconomic and public expenditure management, (iii) ensuring accountability and the transparent use of public funds; (iv) encouraging the growth of the private sector, (v) promoting effective delivery of public services, and (vi) effectively implement a rule of law.
Moreover, the conservation policies should take the issue of population growth as a challenge that calls for pragmatic approaches for its solution. Proactive population policy, education on family planning and implementation of poverty reduction strategies are one of several steps. Furthermore, the issue of HIV/AIDS epidemic in Tanzania needs multi-sectoral intervention because of its cultural, social, economic, political and technological dimensions. Despite the fact that the policy guidelines and strategic framework for the response of HIV/AIDS epidemic and management of its consequences in Tanzania are in place, the reality is that the war against it need efforts geared at ensuring public and private participation. This should be complemented by promotion of the high level advocacy and education, protection of human and communal rights of people infected with and affected by HIV/AIDS. Enhancing health care and counselling of HIV/AIDS patients, ensuring the welfare of the bereaved orphans and survivors of HIV/AIDS, and handling of social, economic, cultural and legal issues, which are related to the epidemic is also important.
Given the negative impacts caused by a number of civil wars that lead to loss of rangelands biodiversity, it is imperative that superior strategies for the conflict prevention and peace building are developed and implemented. Both local and international communities, when necessary, should intervene to fight social vices that lead to civil wars, such as inequalities, corruption and nepotism. There is a need for the establishment of a global network on conflict prevention and peace education in collaboration with the respective ministry of education, civil societies and religious organization.
All development policies, projects or activity should be subjected to Environmental Impact Assessment (EIA) in order to identify their potential impacts. The proactive effort should be made to restore the degraded or damaged range areas, which are preceded by the development activities, such as those in the mining areas as well as in refugee-affected areas.
The problem of climate change and its potential impacts on rangeland biodiversity should be addressed by the adoption of a variety of mitigation and adaptation measures. The measures include limiting or controlling anthropogenic activities such as deforestation, adoption of proper land management practices (including agroforestry), changing energy technologies (e.g. the use of efficient wood stoves and biogas), ensuring proper fire management as well as developing fire reduction strategies for rangelands. Other strategies should involve adopting the integrated land and water management practices, and enhancing synergies between the conservation and sustainable use of biodiversity and climate change. There is an urgent need for the government to assess and identify invasive species and develop effective strategies for their control. This can be done by educating the public about the types of invasive species found in Tanzania and raise awareness of their relevance so that their control can start at the grass root. More research is required to understand vulnerability of different rangeland ecosystems to new invasions by the alien invasive species.
The current conservation approach based mostly on protected area systems is ineffective and limited to protecting species outside protected area. There is dire need for an ‘inclusive conservation approach’ geared towards conserving biodiversity in the wilderness (protected areas), non-protected areas and in urban areas where people live and work (Rija 2010). Most conservation threats emanate from protected area matrices and are conducted by people from urban areas. For example, the rhino killings in the Serengeti National Park by poachers in 2012 were spearheaded by people from cities far away. Extending conservation efforts into non-protected areas including cities would render effective biodiversity conservation countrywide.
We call upon increasing collaborative efforts between local and international scientists in addressing the challenges facing biodiversity conservation across Tanzania’s rangelands. Such efforts should target toward enhancing capacity of local scientists and practitioners particularly in advanced research skills and monitoring techniques of biological resources (Rija and Hassan 2011). These skills are essential to ensuring sustainable conservation of biodiversity especially in wildlife reserves.
This paper was written following a discussion between JRK and AAR on the threats facing Tanzania’s rangelands in the course of teaching Range Ecology and Management course to the undergraduate students pursuing Bachelor of Science degree in Wildlife Management at SUA. We thank two anonymous reviewers for providing comments on the manuscript.